Developments in Peptide Mapping Technology for the Biopharmaceutical Industry

contributed by Thermo Fisher Scientific |
What is peptide mapping?
Peptide mapping is a critical workflow in biotherapeutic protein characterization and is essential for elucidating the primary amino acid structure of proteins.
For recombinant protein pharmaceuticals, such as monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), peptide mapping is used for proof of identity, primary structural characterization and quality assurance (QA).
Global regulatory agencies, including US Food and Drug Administration (US FDA) and European Medicines Agency (EMA), look to harmonized guidelines from the International Council for Harmonisation (ICH). ICH Q6B covers the test procedures and acceptance criteria for biologic drug products, and specifies the use of peptide mapping as a critical quality test procedure for drug characterization used to confirm desired product structure for lot release purposes.
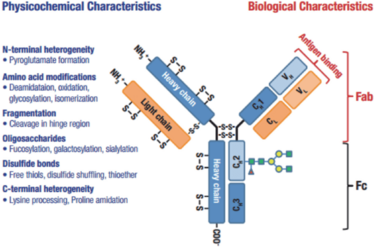
Figure 1: Overview of the typical structure and associated modifications found in a mAb.
In order to generate a peptide map, the therapeutic protein must first be digested into its constituent peptides via a chemical or enzymatic reaction. Robust separation and identification of the resultant peptides then provides insight into a protein’s full sequence information; displaying each amino acid component and the surrounding amino acid microenvironment, including disulfide linkage information. Structural characterization at this level highlights post translational modifications (PTMs) such as site-specific glycosylation, amino acid substitutions (sequence variants) and/or truncations which may result from erroneous transcription of complementary DNA (Figure 1).
Within a bioproduction environment peptide mapping is necessary for manufacturing process monitoring and quality control (QC). It facilitates product comparability testing, which is necessary to identify any productrelated impurities, such as deamidation and/or oxidation following any formulation, manufacturing process or storage change.
Due to its complexity and inherent variability, peptide mapping is generally performed in a comparative manner; for example, biosimilars would be compared to a reference or control substance, such as the innovator biologic, in a side-byside experiment. An in-depth analysis is then required to identify minor and even isobaric differences in protein primary structure.
The modern biopharmaceutical and protein research laboratory is tasked with providing high quality analytical results, often in high-throughput, regulated environments. Some technologies currently employed for biopharmaceutical peptide mapping are subject to:
- high levels of irreproducibility
- poor sensitivity
- high levels of time-consuming manual work – with protracted methodologies that are not amenable to automation and often require 24 hours to achieve full protein digestion.
This variability impacts data confidence. Moreover, it increases potential for introduction of non-product related artefacts during manual sample handling. Outlined herein are the most recent advances in protein sample preparation chemistries, ultra-high performance liquid chromatography (UHPLC), mass spectrometry (MS) hardware and intuitive software for the generation of comprehensive, confident peptide maps.
Log in or register to read this article in full and gain access to The Medicine Maker’s entire content archive. It’s FREE!



















