Keeping a Finger on the Biosimilar Pulse
The rise of biosimilars is great news for overburdened healthcare systems, but it’s a very different proposition to a simple generic. Here, I discuss some of the many challenges – legal, regulatory, non-clinical and clinical – that manufacturers face when developing biosimilar products for global markets.
The first approved recombinant DNA-produced biologic drug was human insulin in 1982. Since then, the global biopharmaceutical market has grown to an estimated $163 billion (Dec 2014) (1), a significant proportion of which is made up of monoclonal antibody (mAb) products. In the EU and US, there are over 30 novel approved therapeutic mAbs, with many more currently going through the application process. The market for these products is forecast to reach nearly $58 billion by 2016 and includes best sellers such as Avastin, Herceptin, Humira, Remicade and Rituxin, which account for over half of all global revenues.
Now, many of the first-generation products, including mAbs, have reached, or are about to reach, patent expiry, which leads us to the advent of biosimilars, defined as legally approved versions of an existing branded biologic, granted marketing approval on the basis of analytical, preclinical and clinical data that show they are highly similar to the reference product. A key driving force for this emerging sector is the universal need for more affordable medicines. Indeed, the potential market for biosimilar products is forecast to be substantial; $64 billion in global biologics sales will be off-patent this year.
Generics versus biosimilars recap
By their nature, small-molecule drugs have simple structures and are synthesized, reproducibly manufactured, and chemically well defined. Together with rigorous testing by originators, this enables generic manufacturers to avoid costly clinical evaluations by establishing bioequivalence to the originator.
However, since biological products are ‘manufactured’ in living systems, the protein expressed from the genetic code will invariably undergo post-translational modifications that alter the structure, giving rise to large, complex molecules, comprising of mixtures of closely related species. The nascent proteins can also be glycosylated – carbohydrates can be attached to the protein backbone to produce a range of glycoforms. The glycosylation pattern will depend on the cell type used and the physiological status of the cell. Many biotherapeutics, including erythropoeitin (EPO), interleukin-2, interferon, granulocyte colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor and tissue plasminogen activator (TPA), occur naturally in the form of glycoproteins. Although there are examples where glycosylation of the recombinant molecule does not appear to have an in vivo influence (for example, G-CSF) in others, most notably TPA and EPO – glycosylation is certainly required for biological activity.
Furthermore, the complexities of the biomanufacturing process and cellular expression systems make exact replication of the originator molecule nearly impossible. Indeed, for the originator, despite years of experience with the patented process, the manufacture of consistent batch-to-batch material may still pose a challenge. As it is not considered possible to manufacture ‘copies’ of bioproducts using a process that will almost certainly be different to the originator, biosimilar proteins cannot be approved as simple generics. Instead, it must be demonstrated that they are similar by performing a side-by-side comparison with a reference sample of the originator molecule, as required by various regions' regulatory pathways.
Regulatory round-up
Back in 2003, the EU first held discussions around the concept of follow-on versions of recombinant proteins and subsequently established the first guidelines, which took effect in 2005, for “similar biological medicinal products” – biosimilars. The European Medicines Agency (EMA) guidelines require physical, chemical and biological characterization of the biosimilar in comparison to the reference product. In addition to this extensive characterization, non-clinical and clinical data are required to demonstrate the same safety and efficacy profiles as the originator. However, the premise is that the amount of non-clinical and clinical data required will be much less than for a novel standalone application.
The initial overarching guideline, CHMP/437/04, was followed by general guidelines on quality and non-clinical/clinical issues. In addition, product-specific annexes were published for a variety of molecules: somatropin (human growth hormone), G-CSF, EPO, insulin, interferon alpha, low-molecular-weight heparins and mAbs. The EMA is continually updating its guidelines, both general and product-specific, as our knowledge grows (2). The first biosimilar approved in Europe (April 2006) was Omnitrope, a version of somatropin, manufactured by Sandoz.
In the US, it is now just over five years since President Obama passed the “Patient Protection and Affordable Healthcare Act” (March 2010). This was the foundation of regulatory legislation designed to cut spiraling healthcare costs by creating a less costly route for approval of certain biotherapeutics. The Biologics Price Competition and Innovation Act (BPCIA), part of the Affordable Care Act, provides a new regulatory approval pathway for biosimilars – the 351(k) route of the Public Health Service Act. This pathway also requires comparison of a biosimilar molecule to a single reference product that has been approved under the normal 351(a) route, with reference to prior findings on safety, purity and potency. One aspect of the legislation unique to the US is the provision for two levels of product –‘Biosimilar’ and ‘Interchangeable Biosimilar’. An interchangeable biological product is one that may be substituted for the reference product without the intervention of the prescribing healthcare provider. Therefore, more data, including clinical switching studies, would be required for a product to be labeled as interchangeable. In addition to the scientific and clinical requirements, there are complex legal and patent procedures to follow, which are not required in other countries.
In July 2014, Sandoz was the first company to announce that it had submitted an application to the FDA under the “351(k)” pathway, for a biosimilar of filgrastim (G-CSF). The original drug, Neupogen, is licensed by Amgen and enjoyed more than $1 billion in sales in 2014. The Sandoz biosimilar was already licensed in the EU in 2008 and marketed as Zarzio in over 40 countries. The FDA approved the product, named Zarxio, in March 2015, for the same indications as Neupogen. However, in doing so, the FDA gave it a designated placeholder non-proprietary name as “filgrastim-sndz”. This re-ignites the ongoing debate on the naming issue – whether the biosimilar name should be unique or the same as the originator drug. At the time of writing, Amgen has been granted an injunction blocking sales of Zarxio in the US market.
In August 2014, Celltrion announced that they had filed an application to the FDA for their product Remsima (a biosimilar infliximab version of Janssen Biotech’s Remicade) – the first mAb to follow the 351(k) route. Celltrion made history with this product back in 2012 when it was the world’s first biosimilar mAb product approved – by the South Korean regulatory authority and then subsequently by the EMA in Europe in June 2014.
In the meantime, many other countries including Brazil, Australia, Turkey, Taiwan, India, Malaysia, Argentina, Mexico, Japan, Canada and South Africa have established regulatory pathways and have licensed copies of biotech drugs. Many countries have also approved biosimilar mAbs such as Remsima, which is approved in 52 countries so far.
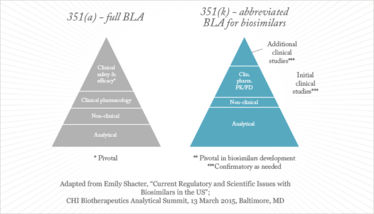
Figure 1. Biosimilars development paradigm.
How similar is similar?
Both the European and US regulatory pathways, and indeed those in many other countries, depend on being able to demonstrate biosimilarity, involving rigorous comparison against batches of originator product, initially at the molecular structure level, then in a step-wise manner in appropriate comparative safety and efficacy tests.
Any manufacturer seeking to develop and market a biosimilar product must conduct comprehensive physicochemical, structural and biological characterization of the (glyco)protein. This task has to be performed at three distinct stages of development: initially to determine the exact sequence of the target originator molecule, then, once the biosimilar product is produced, to confirm its structure, and finally to provide comparative data for the biosimilar versus the originator molecule, as required by regulatory guidelines. Strategies must include assessment of primary and higher-order structure, and batch-to-batch variation should be determined for both the biosimilar and the reference product. This demands the use of multiple orthogonal analytical techniques, utilizing highly sophisticated and sensitive analytical instrumentation. This foundation quality comparison is followed by functional aspects, using binding assays and so on. Only once biosimilarity has been established can we move on to further non-clinical testing and clinical evaluation in the intended population, involving pharmacokinetic and pharmacodynamic Phase I trials followed by clinical efficacy and safety at Phase III as required.
A commonly asked question is: “how similar to the originator molecule must the biosimilar be”? It is clear that the primary protein structure – the amino-acid sequence – must be the same, otherwise it is not considered biosimilar. The guidelines anticipate that minor differences in post-translational forms or product-related impurities may exist and that these should be investigated with regard to their potential impact on safety and efficacy, as it is the total package of data that is taken into account on a case-by-case basis.
Biosimilar drugs are now available in many highly regulated markets, paving the way for more widespread access to biological treatments. Although there are still many questions to be answered, such as extrapolation of indications, interchangeability and naming, the legal and regulatory basis for authorization of biosimilars is built on strong scientific and quality foundations, coupled with appropriate safety and clinical studies.
- R. Otto, A. Santagostino and U. Schrader, "Rapid growth in Biopharma: Challenges and Opportunities", Insights and Publications, Mckinsey.com (2014).
- EMA, “EMA Multidisciplinary: Biosimilars”, www.ema.europa.eu
Fiona Greer is Life Sciences Global Director, Biopharma Services Development at SGS.



















