All Eyes on EMA Approvals
From anti-cancer viruses to H3 blocking narcolepsy drugs – we present a year in European drug approvals
The European Medicines Agency (EMA) recently released its “highlights of 2015” in terms of new medicine approvals. All in all, it seems that 2015 was a good year with 93 medicines recommended for marketing authorization, including 39 new active substances. In 2014, on the other hand, just 82 new products were recommended for approval. Our infographic brings you up to speed with some of the key approvals from 2015 that will likely be coming to a European market near you soon.
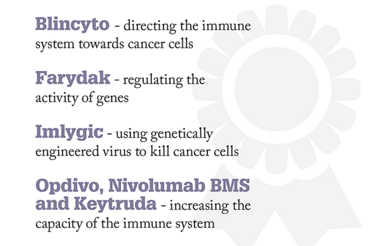
Cancer Approval Highlights:
Bringing Medicines to Patients Faster

Safety First
Inspections were conducted in 62 countries worldwide
>2,590 GMP inspections. >270 GCP inspections.
>190 pharmacovigilance inspections.
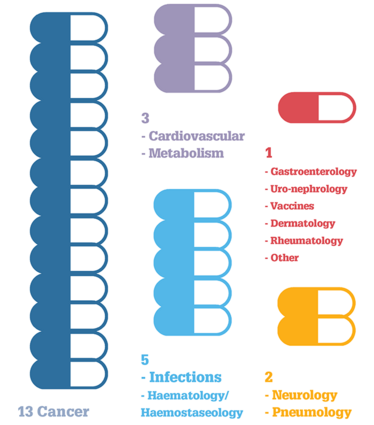
Medicines with New Active Substances in 2015

New Medicines for Rare Diseases: 18
Highlights:
Blincyto for patients with acute lymphoblastic leukemia
Farydak for patients with multiple myeloma
Hetlioz for blind adults with sleep-wake disorder
Kanuma for patients with lysosomal acid lipase deficiency
Kyprolis for patients with multiple myeloma
Lenvima for patients with thyroid cancer
Strensiq for patients with childhood hypophosphatasia
Unituxin for patients with brain cancer (neuroblastoma)



















