Easy as eCTD?
Deadlines for mandatory eCTD transitioning are approaching – and companies need to understand how to prepare a compliant submission.
The pharmaceutical market is one with high rewards. It has been reported that pharmaceutical spending growth should match health spending growth at an average of 4.3 percent during 2015- 2019, and global pharmaceutical sales should reach $1.4 trillion (€1.18 trillion) by 2019. Biotech drug sales reached an estimated $289 billion (€244 billion) in 2014 and are projected to grow to $445 billion (€375 billion) by 2019. In addition, biotech’s share of worldwide prescription drug and over-the-counter sales is projected to increase from 23 percent in 2014 to 26 percent in 2019 (1).
On average, it takes around 12 years for a new drug to go from invention to market. During this time, a tremendous amount of information about the molecule will be collected. If a drug is ultimately successful in clinical studies, the pharma manufacturer will seek regulatory approval via a Marketing Authorization Application (EMA) or New Drug Application (NDA), which should demonstrate the analysis of data obtained during development. These applications are complex, lengthy documents; in the 1990s, a typical MAA consisted of at least 100,000 pages. To standardize the application dossier, the Common Technical Document (CTD) was developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), in collaboration with the EMA, FDA and Japanese Ministry of Health, Labor and Welfare.
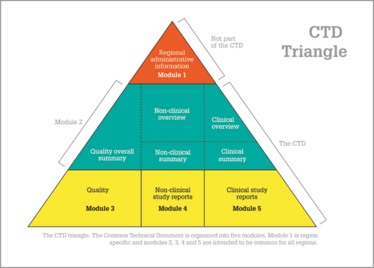
The CTD describes the organization of modules, sections and documents to be used by an applicant for the marketing authorization of a medicinal product for human use in the three regions that are party to the ICH. Figure 1 demonstrates the five models of CTD: region-specific information, summary documents, quality-related information, non-clinical study reports, and clinical study reports. The CTD is defined as an interface for industry-to-agency transfer of regulatory information, while at the same time taking into consideration the facilitation of the creation, review, lifecycle management, and archiving of the submission.
It is fair to say that CTD has revolutionized regulatory submissions – primarily because the standardization means there is no need to reformat the lengthy information for submission to different regulatory authorities. In July 2003, the CTD became the mandatory format for new marketing authorization applications to the EMA and Japanese regulator, the PMDA – and the strongly recommended format of choice for NDAs in the US. But today, we live in the electronic age, so it is only natural for regulatory submissions to move to an electronic format – enter the electronic Common Technical Document (eCTD).
For a number of years now, applicants seeing marketing authorization for a drug product have had the option of submitting an eCTD in parallel with a paper CTD submission, but regulators now want to make most submissions electronic only. Many of the deadlines for moving to electronic submissions have already passed, but others are approaching rapidly.
Benefits of eCTD
- Improved reviewer efficiency
- Reduced time to approval
- Submission via Electronic Submissions Gateway (ESG) (US) and Common European Submission Platform (CESP) (Europe) enable immediate receipt by the regulatory body
- Improved handling and archiving of submissions (both sponsor and regulatory body)
- Search functionality and increased tracking ability
- Accessibility of documentations across modules
- Ability to re-purpose documents for submission in other regions
- Simplified lifecycle management
- Avoids duplication of the information within the application
Embracing the electronic way
In the US, the eCTD is now the standard format for submitting applications, amendments, supplements, and reports to the Center for Drug Evaluation and Research or the Center for Biologics Evaluation and Research. All submissions must be transferred to eCTD format for investigational New Drug Applications and Drug Master Files from 5 May 2018, and all other applications must be submitted via eCTD or they will not be filed by the national regulators, including FDA or MHRA. For the most part, the US industry has embraced eCTD. Since the introduction of eCTD, submissions to FDA using this format have grown each year. In fiscal 2007, eCTD submissions made up about nine percent of NDAs; in fiscal 2016, eCTDs accounted for 93 percent of NDAs.
In Europe, the picture – and uptake of eCTD – has been somewhat different. Out of 28 National Competent Authorities in Europe, 21 still accept paper submissions for national applications. Although the use of paper submissions is relatively rare, some manufacturers use Non-eCTD electronic Submissions (NeeS) or a single pdf-file – neither of which are ICH standard. A number of initiatives have been undertaken to improve electronic submissions within the region. For instance, the EMA has required mandatory eCTD for applications of Centrally Authorized Products for human use from 2010. The agency has also developed structured electronic Application Forms (eAFs) and worked with the Heads of Medicine Agencies to set up a Common European Submission Portal (cesp.hma.eu) to facilitate the move to eCTDs. The initiatives have been developed with the support of the European pharmaceutical industry because of the benefits that standardization and eCTD offer – in particular, eCTDs promote open international standards and interoperable systems that support the exchange of data and documents, thus easing the regulatory process
for companies.
There was a need for Europe to establish a clear roadmap that would remove the need for paper and physical electronic media to enable the pharmaceutical industry and regulatory authorities to plan for necessary investments and organizational The aim behind this has been to help to improve efficiencies, reduce the administrative load and increase transparency via a more streamlined approach to electronic processing of information. Its intended purpose is also to allow for the availability of all information electronically to both the authorities and industry in a single source.
Mandatory eCTD is required for all new MAAs submitted under the National Procedures (NPs) by July 2018 and for all regulatory activities affecting NPs from January 2019. Final guidance for the initial implementation of the electronic submission requirements includes new drug applications (NDAs), abbreviated new drug applications (ANDAs), certain biologics license applications (BLAs), master files and for commercial investigational new drugs applications (INDs). The schedule indicated that NDAs, BLAs and ANDAs should be submitted electronically in eCTD format starting on May 5, 2017 (May 5, 2018 for commercial INDs) but the submission of master files in eCTD format was extended by one year to May 5, 2018 in response to industry pressures and internal reviews. Exempted submissions only include non-commercial INDs which refer to products that are not intended to be distributed commercially as well as investigator-sponsored INDs and expanded access INDs. However, while these are an exception to the rule, submissions in eCTD are still accepted.
Most European organizations are now well on their way to embracing eCTD – which will ultimately reduce the workload for regulators and help them to function more efficiently. Hopefully, this will result in faster review of submissions and reduced time to market.
A Smooth Transition
I was recently involved in helping West Pharmaceutical Services make the transition to eCTD. West maintains an extensive Drug Master File (DMF) portfolio with the FDA and Health Canada for its products and processes. The company issues more than 1,500 Letters of Authorization to its customers annually and its elastomer formulations DMF is one of the most heavily accessed master files held by the FDA. Given this, the agency was very amenable to seeing these DMFs transitioned to eCTD, and had discussed the possibility with West on several occasions.
West initially developed a strategy document detailing necessary steps, required deliverables and critical interdependencies before evaluating existing in-house document management platforms and external electronic publishing options. The team then identified additional software requirements to establish a robust electronic submission management system. For document authoring and management, the firm assessed an existing platform to determine whether it could be sufficiently customized to support its requirements, including versioning and document approval for traceability; meeting agency PDF document security specifications; and maintenance of document integrity. West evaluated three options for its electronic publishing needs: software purchase, Software-as-a-Service (SaaS) and outsourcing. Each option was evaluated against the estimated submission workload to compare cost effectiveness. Ultimately, West selected the SaaS option as the best choice based on their need for flexibility and ability to accommodate its specifications.
Without a conversion method for implementing the database solution, West decided to engage both the FDA and Health Canada in a collaborative effort to define best practices moving forward. It was a unique opportunity to build and refine DMF submission requirements and it was in the mutual interest of West, its customers, and the FDA to optimize the submission process.
In the collaborative discussions that followed, West and the FDA identified and addressed challenges in the former process, as well as challenges specific to managing eCTD DMFs in the process moving forward. For example, Letters of Approval (LOAs) have always been processed using paper copies at the FDA and managed separately from the DMFs. However, with the move to eCTD, both West and the FDA will manage LOAs electronically as part of each DMF, which required a new management process.
West submitted five DMFs in eCTD, including four conversions and one new submission. Each DMF can now be submitted to the FDA centers and Health Canada. By using eCTD for the DMF, customer feedback was positive with a direct benefit to their overall submission process, including a simplified, streamlined review experience.
Making the move
For companies that have not yet embraced eCTDs, the clock is ticking. Transitioning from NeeS to eCTD submissions requires time and financial investment. And, in my experience, manufacturers should expect preparation of their first eCTD submission to take between six to 12 months. This transition will require infrastructural changes at an organizational level. One of the main challenges for manufacturers is their own regulatory infrastructure and how submission documents will be processed and archived. Companies also need to choose an electronic publishing option to meet their needs. They must create, authorize and share documents electronically, and must adopt electronic document creation and processing for the information that needs to be submitted in the application.
Setting up an eCTD platform requires not just a change in the document creation and maintenance practice, but also procurement, set up and validation of the eCTD software and other associated tools, such as the validator. Companies also need to be mindful about the compatibility of these software options. These specialized software options require technical expertise to handle and, thus, recruitment of experts and training of users is required. Setting up clear workflows and written procedures is a must to reduce variability between different parties within the company.
Preparation ahead of these deadlines is key as this will position a company well against its competitors. Equally, by having a strong regulatory resource, this will enable the company to grasp a deep understanding of the submission requirements, which will set the foundation for further understanding of the technical skills and regulatory requirements necessary to file compliant eCTD submissions. In addition, readdressing best practices for the use of programs such as MS Word and Adobe Acrobat in preparing content for eCTD will not only ensure increased efficiencies but also reduce the risk of delays for future eCTD filing.
All of this time and effort will ultimately be rewarded; moving to eCTD will ease the regulatory review process, minimize back-and-forth with the agency regarding submission content and quality, and improve the chances of approval during the first review cycle. Whether manufacturers are preparing to transition to eCTD in Europe or the US, the countdown has begun; companies must get to grips with the new requirements.
Pallavi Trivedi is a regulatory consultant at Morningside Healthcare, UK, and an active member of the Regulatory Affairs Professionals Society (RAPS) and the RAPS European Council (REC).
- Deloitte, “2016 Global life sciences outlook”, (2015). Available at: bit.ly/DeloitteOutlook. Accessed March 2, 2018.
- EMA, “European Medicines Regulatory Network eSubmission Roadmap,” (2017. Available at bit.ly/2p6bS6n. Accessed March 9, 2018.
Pallavi Trivedi is a regulatory consultant at Morningside Healthcare, UK, and an active member of the Regulatory Affairs Professionals Society (RAPS) and the RAPS European Council (REC).



















