Good Things Come in Small Packages
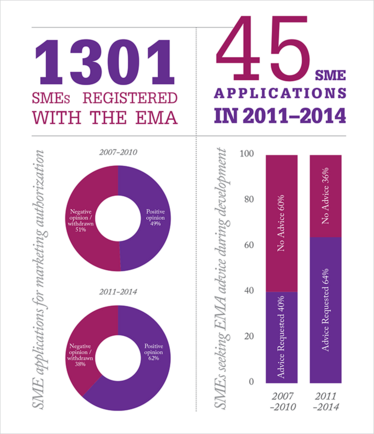
Increased uptake of the EMA’s advisory services, including scientific advice during development and biomarker qualification, seems to have led to increased success rates for small and medium-sized enterprises (SMEs), according to the EMA’s annual SME Report. The report notes, “Initiating dialogue early and repeating it at major milestones is important to decrease the quality and clinical failure rate at time of marketing authorization review.” Notably, there are still areas for improvement, with quality and clinical documentation attracting the most objections – particularly in the biologics area.

As an Editor at Texere, I’m working closely with our audience to create vibrant, engaging content that reflects the hard work and passion that goes into bringing new medicines to market. I got my start in biomedical publishing as a commissioning editor for healthcare journals and have spent my career covering everything from early-stage research to clinical medicine, so I know my way around. And I can’t think of a more interesting, challenging or important area to be working in.



















