Mylan’s Media Storm
Mylan introduces new schemes to help tackle EpiPen costs for patients, as well as plans for a new direct-to-patient distribution model
Following a media frenzy around the increasing costs of Mylan’s EpiPen, Mylan has taken action: the company is doubling eligibility for its patient assistance program and offering a new savings card for up to $300 (1). In addition, the company is looking to open a pathway that will allow patients to order EpiPen directly from Mylan to avoid the costs associated with high deductible health insurance plans.
Mylan became the center of media controversy after it was revealed that the price of its EpiPen, used for treating anaphylaxis, has increased by up to 500 percent since Mylan acquired the drug in 2007 – in some cases costing patients over $600 for a pack of two EpiPens (patients are recommended to carry two pens at any time). The company has strongly advocated for increased awareness of the dangers of anaphylaxis and the benefits of EpiPen, particularly to parents and schools, and much of the media attention has focused on the perception that children’s lives are being put in danger because some parents can no longer afford the treatment.
Mylan blamed the increasing price to patients on changes to the US healthcare insurance landscape. “With the current changes in the healthcare insurance landscape, an increasing number of people and families have enrolled in high deductible health plans, and deductible amounts continue to rise,” the company explained in an August 22 statement (2). “This current and ongoing shift has presented new challenges for consumers, and now they are bearing more of the cost.”
In its latest statement, dated August 25, Mylan says it is committed to the following actions (1):
- Offering a savings card for up to $300 that will effectively reduce the cost exposure by 50 percent for patients who would have otherwise paid the full list price.
- Doubling eligibility for the company’s patient assistance program to 400 percent of the federal poverty level. This means a family of four making up to $97,200 will pay nothing out of pocket for EpiPen.
- Continue to offer the EpiPen4Schools program. The program was launched in August 2012 and has provided more than 700,000 free auto-injectors and educational resources to more than 65,000 schools nationwide.
- Open a pathway so that patients can order EpiPen direct from the company.
In addition, the company reveals details about the economic supply chain, showing why patients are paying more for EpiPen because of the US health insurance system (see Figure 1).
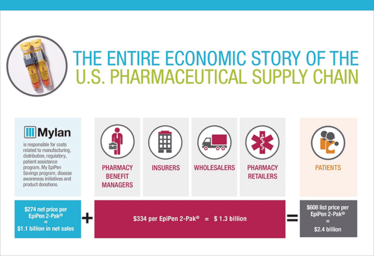
Figure 1
How the US public reacts remains to be seen. The revelation of the price hike has led to comparisons between Martin Shkreli, who raised the price of Daraprim by over 5000 percent in 2015, and Mylan’s CEO, Heather Bresch, as well as an enormous number of aggressive comments on social media from angry patients. US Senators have also been demanding an explanation.
In the new statement, Bresch said, "We recognize the significant burden on patients from continued, rising insurance premiums and being forced increasingly to pay the full list price for medicines at the pharmacy counter. Patients deserve increased price transparency and affordable care, particularly as the system shifts significant costs to them… All involved must also take steps to help meaningfully address the U.S. healthcare crisis, and we are committed to do our part to drive change in collaboration with policymakers, payors, patients and healthcare professionals."
If you are interested in the US landscape for medicines, look out for our upcoming September issue, where we focus on how the presidential elections in November could impact the pharma industry – and the prices of medicines.

Heather Bresch
Bresch was featured in The Medicine Maker’s Power List in April 2016. The Power List is an annual celebration of the most influential individuals involved in drug development and manufacturing, as nominated by readers and selected by an anonymous judging panel. Bresch was #1 on the list, with one judge saying: “Mylan and Heather Bresch have been involved in a lot of drama in the past 12 months, including fighting a hostile takeover bid from Teva and then failing to acquire Perrigo in Mylan’s own hostile takeover attempt, but she is also undoubtedly a powerful leader and has greatly influenced the generic drug industry, among other things.”
The Power List will be published again in April 2017.
- Mylan, “Mylan Taking Immediate Action to Further Enhance Access to EpiPen® (Epinephrine Injection, USP) Auto-Injector”, (2016). Available at: bit.ly/2bkTxtg. Last accessed August 25, 2016.
- Mylan, “Mylans Commitment to EpiPen (epinephrine injection, USP) Auto-Injector Access”, (2016). Available at: bit.ly/2bU6uyV. Last accessed August 25, 2016.

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















