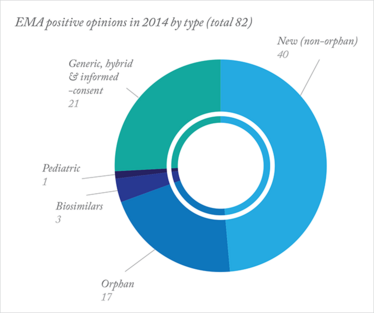
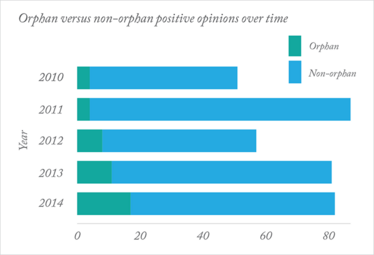
Judging from recent statistics from the European Medicines Agency (EMA), perhaps so. A record number of positive opinions for medicines for rare diseases were issued by the EMA in 2014: 17 positive opinions (compared with just 11 in 2013), including the first medicine for Duchene muscular dystrophy (Translarna; PTC Therapeutics) and the first treatment for erythropoietic protoporphyria (Scenesse; Clinuvel). 2014 also saw another first, with the EMA recommending a therapy based on stem cells. Eight new medicines for cancer were also recommended. Overall, the EMA recommended 82 medicines for approval in 2014.

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.