Advancing Therapies
A report highlights the acceleration of cell and gene therapy approvals worldwide
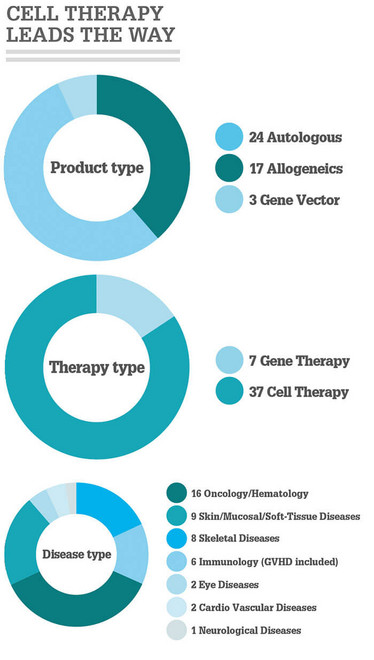
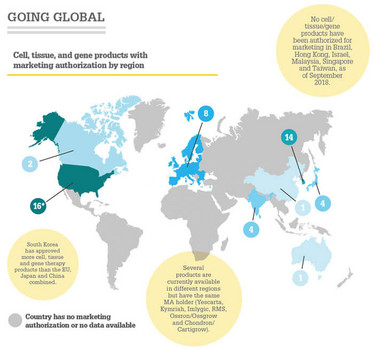
The International Society of Cell and Gene therapy (ISCT) has published a snapshot of advanced therapeutic approvals throughout the world (1). ISCT intends the report to be a “living document” – periodically updated and linked to a dedicated section of the ISCT website. Products authorized from 2015 to September 2018 represented 45 percent of all cell, tissue and gene therapy approvals worldwide – highlighting the growing number of marketing authorizations granted by regulators. The US had the greatest number of approved cell, tissue and gene therapies, with 16 (eight products included in this number, however, are based on cord blood hematopoietic progenitors for unrelated donor hematopoietic progenitor cell transplantation; similar products are available in most countries as cell transplants and not as marketed products).
“The aim is to ultimately inform, by periodic snapshots, the scientific community, healthcare stakeholders and patient associations on authorized cell and gene therapies (CGT) as a way to increase communication around the approved therapeutic approaches charged with heightened expectations,” said the authors of the report. “This article reflects the dynamic momentum around authorized CGTs coinciding with the parallel increase of unproven approaches where cells are delivered without appropriate and rigorous scientific and regulatory assessment and authorization.” Our infographic provides an overview of the key facts and figures.

- N Cuende et al., “Cell, tissue and gene products with marketing authorization in 2018 worldwide,” Cytotherapy, 20, 1401 1413 (2018). PMID: 30366616.

Over the course of my Biomedical Sciences degree it dawned on me that my goal of becoming a scientist didn’t quite mesh with my lack of affinity for lab work. Thinking on my decision to pursue biology rather than English at age 15 – despite an aptitude for the latter – I realized that science writing was a way to combine what I loved with what I was good at.
From there I set out to gather as much freelancing experience as I could, spending 2 years developing scientific content for International Innovation, before completing an MSc in Science Communication. After gaining invaluable experience in supporting the communications efforts of CERN and IN-PART, I joined Texere – where I am focused on producing consistently engaging, cutting-edge and innovative content for our specialist audiences around the world.



















