
Exploring Hot-Melt Extrusion
When it comes to developing a viable formulation for an API with poor solubility and poor bioavailability, manufacturers should look to hot-melt extrusion (HME) – a well-established process that is easy to scale up with the right mathematical models
Sampada Upadhye | | Longer Read

This article is part of our special focus on "traditional" pharma: The Small Molecule Manufacturer (read more here). You can find more articles from The Small Manufacturer here.

First developed for polymer processing in the plastics industry in the 1930s, hot-melt extrusion (HME) is a well-established and flexible technology in the pharma industry. HME facilitates the formulation of low-solubility, low-permeability drugs into a number of patient-friendly dose forms, including tablets, capsules or free-flowing granules.
HME uses heat and pressure to melt a polymer before it is forced at constant pressure through an orifice (see sidebar: “How HME Works”). The resulting “extrudate” is then further processed into products that display uniform API particle distribution and density. HME has become widely used in drug product development, because of the added flexibility it offers; by selecting suitable polymeric matrix excipients, formulators can create products that achieve higher bioavailability (than traditional formulations) as well as targeted delivery into the upper regions of the intestine, when drugs have poor solubility and/or poor permeability.
Poorly-soluble APIs are a key challenge for the development of solid dosage forms. The more efficiently the active ingredient can be released from a specific delivery system into the bloodstream, the better its bioavailability. It is estimated that up to 90 percent of all newly-synthesized APIs are poorly soluble and almost half of drug development project failures are due to a lack of API solubility and the resulting poor drug bioavailability and/or variable pharmacological behavior (1).
Dissolution rate is also important for the absorption of orally ingested substances; a drug is practically unavailable if the dissolution time of an active is longer than its gastrointestinal transit time. The dissolution rate itself is not only determined by the geometry and the surface of the particles, but also by the concentration difference between the saturation concentration and the actual solution concentration. This difference can be very small for poorly soluble compounds, so the surface and the geometry of the particles become the most relevant parameters for the dissolution process.
For APIs exhibiting solubility-limited absorption, solid dispersions and solutions can be obtained in a number of ways using solution processes in which materials are dissolved in a suitable solvent and then converted into amorphous form using spray drying, freeze drying, casting techniques, or by using melt processes. There are several major processing advantages of HME:
- HME is a solvent-less process
- The HME process is independent of the compressibility characteristics of the starting material
- HME is a continuous, well-controlled process based on co-rotating twin screw technology that offers good blending characteristics, resulting in an API that is homogeneously dispersed or dissolved in the carrier matrix
- The HME process is unaffected by any crystalline modifications of the API because it is based on amorphous solid dispersion in a carrier matrix that provides flexibility in downstream processing and the ability to produce a range of formulation types
- HME allows the introduction of taste-masking techniques.
For the HME approach to be successful, the time in which the systems can be cooled down to temperatures below the glass transition temperature (Tg) is of great importance – a factor that also affects aggregation and crystallization of the API. Drug formulations with targeted release kinetics are achieved by a molecular dispersion of the active substance in the carrier matrix. The choice of process parameters and the screw design determine the quality of the extrudate; small amounts of active ingredient are sufficient to evaluate the suitability of an HME process with regard to processability and quality characteristics of the extrudate. A systematic approach to HME based on an understanding of the interplay between process and product enables the development of stable amorphous solid dispersions, and the rapid scale-up of clinical manufacturing.
Points to remember
To develop suitable formulations, it is beneficial to keep the extrusion formulation process as simple as possible through the choice of a suitable polymer. The use of a plasticizer for improved processing should only be used if necessary, and a solubilizer will be required for improved solubility and prevention of recrystallization when in the presence of gastric and intestinal fluids.
Selection criteria for the polymeric carrier to be used in the HME process include its interaction with the drug; the potential for supersaturation; and the solid-state solubility of the drug in the carrier. Important polymer characteristics include the design of the manufacturing process, the solubility of the polymer in water and organic solvents, its molecular weight and its glass transition temperature. Ideally, the polymer matrix acts as a solid solvent and the drug is molecularly dissolved. To achieve this, the polymer should have good thermoplastic behavior (deformability is essential); thermal stability (Tg from 50 to 200°C); low hygroscopicity to prevent crystallization; and no toxicity (so that it may be used in large amounts).
A number of analytical techniques are used to control the quality of the extrudate, including optical microscopy to determine sample surface morphology and the presence of crystalline particles; scanning electron microscopy (SEM) for higher-resolution imaging; and atomic force microscopy (AFM) to provide three-dimensional images. Differential scanning calorimetry (DSC), X-ray diffraction crystallography (XRD), solid-state nuclear magnetic resonance (ss-NMR), and IR and Raman spectroscopy can also be used to analyze the quality of the API/polymer extrudate.
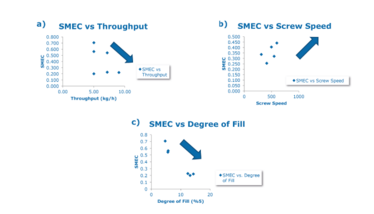
Figure 1. Trends in the relationship between SMEC and a) throughput b) screw speed c) degree of fill when run on a 27-mm TSE.
The QbD approach
HME enables product development driven by Quality-by-Design (QbD). Parameters, including the degree of dispersion, level of impurities, extent of dissolution, stability and morphology, can be optimized through the application of an extrusion process in which characteristics such as residence time and shear stress are controlled via a number of input variables. These include feed rate, temperature, screw design, screw speed and the physical properties of the materials.
We have developed a mathematical approach to the scale-up of HME from laboratory-scale to production-scale for the manufacturing of a drug for a phase II program. The approach (OptiMelt Plug & Play) predicts HME process-independent parameters. Specific mechanical energy consumption (SMEC) is the critical process response in the scale-up of the HME process. It is defined as the energy supplied to the material by the extruder motor and is dependent on the screw configuration and speed.
SMEC can be used to predict critical process parameters (CPPs), such as screw speed and feed rate, and degree of barrel fill to ensure successful scale-up.
Equation 1. n= screw speed (rpm), τ= Torque [Nm], m= throughput [kg/h]
Converting the SMEC from the laboratory-scale extruder to larger-scale extruders via the calculation model identifies the new process parameters. The predictive model also provides an estimate of product quality and helps ensure the set quality target product profile (QTPP) is met for both small- and large-scale batch sizes.
In the below example, the application of the SMEC calculation model from laboratory-scale studies aided in the creation of a design space to produce a stable amorphous indomethacin extrudate during the scale-up process (see Figure 1).
The CPPs (screw speed and feed rate) can be optimized to achieve the desired scale-independent responses, and maintained within the specified design space for efficient manufacturing (see Figure 2). Scale-independent process response, such as SMEC, is ideal for associating the quality to the process. Changes in equipment size, brand, and/or location require the determination of the impact that these factors may have on the CQAs of the process inputs, if they are to ensure a successful tech transfer of an HME process.
At the end of laboratory-scale development, not only could the clinical trial supply be manufactured, but it is also possible to make a sound risk assessment that supports further scale-up by using a reasonable amount of API.
An integrated end-to-end HME process platform provides the capability to formulate, develop and commercialize differentiated final dosage forms. It enables a holistic approach to bioavailability enhancement by broadly addressing multiple bioavailability factors, including optimization of product efficacy, safety and release properties. Beginning with initial API and preformulation studies, the required formulations can be developed through the application of a range of screening technologies and analyses.
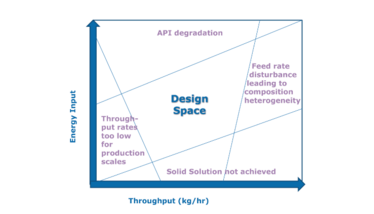
Figure 2. Process inputs and response factors must be well characterized to identify the design space for specific product attributes and manufacturing outputs.
Formulation flexibility
Although twin-screw hot melt extrusion has been more popularly used for manufacturing amorphous solid dispersions, twin screw extruders are versatile pieces of equipment. They are also used for continuous manufacturing of immediate release and controlled release products of crystalline API. Based on the target product profile of API, various extrusion granulation techniques, such as wet, dry and melt granulation approaches, can be employed to yield appropriate immediate release and controlled release product.
In summary, bioavailability enhancement and dose form flexibility can both be achieved through the application of HME technology. Moreover, the wide range of dose functionality achievable allows immediate and controlled-release formulations to deliver more active drug, when and where it is needed in the body.
How HME Works
The extrusion process combines conveying, melting, blending, kneading, and degassing into a continuous process, and the various substances involved are continuously added into the processing section or “barrel” of the extruder in a precisely controlled manner. Co-rotating screw shafts regulate material transportation and mixing, and determine the residence time in the various zones. Different temperature profiles can be applied in each zone to meet the processing needs of the polymer and the API, while air or other gases are removed from the molten mass using a vacuum unit to guarantee a bubble-free discharge of molten extrudate through a nozzle at the end of the processing section. A solid product is formed upon cooling of the molten extrudate, and this can be used in downstream processes to develop the desired solid oral dosage form.
Twin-screw extruders, which are predominantly based on segmented screws, can be configured in various ways to meet different conveying, kneading and blending requirements. The important parameters of the screw elements are the element pitch and the number of flights, as well as the outer and inner diameter of the screws. Pitch affects the residence time of the material in the processing unit, while the material flow rate depends on the number of flights in the screw elements. The torque transferred to the screw determines the material throughput.
Mixing and compensation of concentration differences is achieved by the mixing elements of the equipment, such as kneading blocks: staggered kneading discs hinder the flow of the extrusion mass along the main conveying direction, while refracted and redistributed material flow results in a distributive mixing effect. In addition, the use of reverse-conveying screw elements improves intermixing. Thus, the ability to arrange the extruder’s screw elements in almost any combination allows the extruder to be adapted for almost any extrusion task.
For the extrusion of APIs, barrel temperature, screw speed and feed rate are the most important process parameters. Low screw speeds result in a long residence time of the material in the extruder, potentially leading to an increase in degradation and impurities, while high screw rotation speeds may cause incomplete mixing and physical instability resulting in recrystallization of an amorphous API.
The shaping of the molten mass occurs when passing under pressure through a die with a narrowed cross-section, and the die flow channel must be aerodynamically designed to allow proper melt flow to be achieved. In addition, the elasticity of the polymer melt and its cooling rate after passing through the die are important – at this stage, the pressure and temperature should be constant to obtain a uniform product.
Solid carriers, excipients and APIs are dosed either individually or are combined in a precisely controlled mixture for dosing, with gravimetric dosing systems being preferred over volumetric dosing systems in pharmaceutical applications. Liquid or gaseous components may be introduced directly into the melt through side feeding devices.
- T Loftsson, ME. Brewster, “Pharmaceutical applications of cyclodextrins: basic science and product development,” J Pharm Pharmacol, 62, 1607-1621 (2010).



















