
Hidden Analytical Advantages
Contract laboratories need to be treated as equal partners rather than service providers to optimize the benefits of outsourced pharmaceutical analysis
Greg Thiele | | Longer Read
How do you choose which properties to measure when it comes to characterizing APIs, excipients, or even the formulation? The identification of critical quality attributes (CQAs) and critical material attributes (CMAs) is an integral component of Quality by Design (QbD) but decisions around how best to measure them are not always easily made in-house. QbD places emphasis on process and product understanding, and is intended to reduce risk. Though potentially demanding, it facilitates a more flexible regulatory approach – an important consideration when putting together the robust submission package required. The knowledge accrued is also helpful for ensuring secure optimization of the supply chain (1). And beyond CQAs and CMAs may lie many additional variables – powder flowability, porosity and specific surface area, for example, depending on the pharmaceutical product type, can affect development and manufacturing and are, therefore, valuable to measure.
The challenges are significant, so many companies choose to outsource their analytical testing. In fact, in 2018, the global pharmaceutical analytical testing outsourcing market was valued at $5.59 billion and is set to grow by 8.1 percent over the next seven years (2). The accrual of savings holds value for many as the need to invest in and use new instrumentation and train staff falls away. Choosing a good contract laboratory or analytical service gives you access to a broad range of equipment as well as in-depth expertise.
Though companies hold the expectation that outsourcing partners will be able to provide high-quality data in a closely specified timeframe – at competitive prices – a question remains: how do you develop a relationship that best draws upon the strengths of both parties? In my experience, making broad requests of a contract lab offers their experts greater scope to contribute as an equal partner in the research process. Rather than dictating conversations with requests like, “I’d like to measure the particle size of these granules by laser diffraction”, companies should consider making more open-ended requests such as, “I’d like to characterize these granules to develop correlations between their properties and those of tablets manufactured from them”.
The risk of making such requests, however, is that that they may drive up the cost of outsourcing. But establishing a more comprehensive package of measurements for APIs or formulation is entirely consistent with the QbD approach and can pay dividends throughout the lifetime of a product. In reality, demonstrating a robust product understanding not only underpins a successful submission but also lays the foundation for the effective supply selection and process troubleshooting needed for long-term economic manufacture.
Mercury intrusion porosimetry is a good example of a technique that may be excluded from a narrowly prescribed analytical schedule, but can add value to more comprehensive characterization of granules for tableting and/or finished tablets (3). This core physical technique robustly quantifies internal structure, but tends to be underused by the pharmaceutical industry because of the health and safety concerns associated with working with mercury and a lack of understanding of the benefits of the technique. Casting a wider analytical net can also provide solid evidence of a company’s understanding of the factors affecting the integrity of critical measurements. For instance, as shown in Figure 1, particle size (an important variable for many pharmaceutical products because of its influence on characteristics such as bioavailability and dissolution behavior) can be measured using several techniques. But each technique has different strengths and weaknesses, which make it well suited to a particular application – or ill suited to another (4).
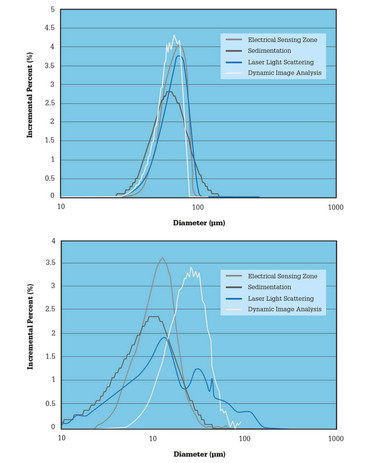
Figure 1: A change in particle shape from spherical (left) to rod-shaped (right) creates a divergence in the particle size distribution data reported by different particle sizing techniques.
Spotting superior supplies
Analytical data is also important when considering the supply chain. The supply chains of the modern pharmaceutical industry are global and complex, and though drugs under patent enjoy some protection with respect to their ability to make profits, generic competition creates fierce pressure, cutting the prices of many drugs. Being able to quantify the potential of a particular supply chain makes it possible to choose the premium products that will stand the test of time and deliver in terms of profitability. One factor that directly impacts the value of supply for specific processes is the fact that certain bulk powder properties like compressibility or flowability are rarely included, or measured, in a formal specification or market contract. Powder testing is an area that has seen considerable advances in the last decade, and those leading the way in its application routinely use multi-faceted characterization based on dynamic, shear and bulk property measurement to maximize insights. Experience suggests that this approach can be highly productive in identifying materials that will exhibit superior process performance from supplies that seem relatively similar (5).
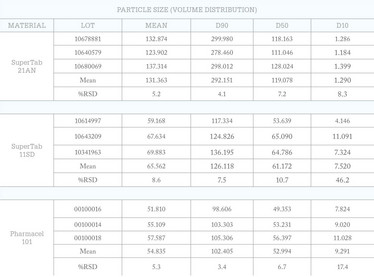
Table 1: High sensitivity laser light scattering analysis picks up significant variability in the level of fines in the three excipients (all results reported in microns).
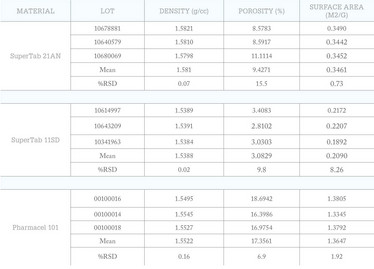
Table 2: Adding less routinely measured parameters into the characterization mix can be a relevant and valuable way to differentiate suppliers.
Tables 1 and 2 show data measured for commercially available excipients: anhydrous lactose (SuperTab 21AN), spray-dried lactose (SuperTab 11SD), and microcrystalline cellulose (Pharmacel 101) (all DFE Pharma). These provide further insight into how supplies marketed under the same specification can be usefully differentiated on the basis of superior analytical data. These excipients are highly likely to be associated with a particle size specification, but the static light scattering system used for the measurements (Saturn Digisizer II, Micromeritics) delivers higher resolution than most commercial systems. This is achieved by using a charge coupled device to detect the light scattering pattern produced by the sample, rather than the photo diode array deployed by standard instruments. The results suggest that there is considerable variability in the particle size of the supplies, most notably in terms of the D10 figure – the level of fines. Where fines impact process performance, high sensitivity particle size analysis may, therefore, be useful in identifying a superior supply.
Table 2 shows the results of applying an extended range of material characterization techniques to measure properties which, unlike particle size, may not be routinely considered. These include density (helium pycnometry), porosity (mercury intrusion porosimetry), and specific surface area (gas adsorption using krypton gas). These results show that all three substances exhibit relatively high variability with respect to porosity; SuperTab 11SD also exhibits a relatively high %RSD for specific surface areas. These properties may be neither measured, nor controlled during manufacture, but they may be relevant to a specific application. For example, the specific surface area of magnesium stearate has been securely correlated with the dissolution performance of tablets (6). Magnesium stearate is routinely used in very low concentrations to improve the flow properties of tableting blends, and more generally as a lubricant to enhance the efficiency of tableting processes. It is a naturally sourced excipient, manufactured via a range of different processes, and supplies on the open market consequently exhibit significant variability.
In an API, higher specific surface area is usually associated with faster dissolution but for flow additives such as magnesium stearate the situation is less straightforward. The mechanisms by which magnesium stearate enhances formulation behavior are complex, but the use of sources with a higher specific surface area has been associated with a slow-down in the dissolution of certain APIs (6). This effect may be attributed to more effective coating of the API by the hydrophobic magnesium stearate. Recent research has also identified particle morphology as affecting the impact of magnesium stearate on dissolution performance (6). When choosing between magnesium stearate supplies, a rigorous physical profile is essential to efficiently avoid one that could detrimentally affect product quality.
The right advice
Outsourcing analysis is becoming an increasingly popular decision, but success relies on developing fruitful working relationships with contract laboratories that recognize and draw on the strengths of each partner they work with. Though there is a place for closely prescribed, narrowly defined “analysis to order,” an exchange of expertise will add value to the working relationship. For example, many pharma companies wouldn’t necessarily appreciate the extent to which analytical data could valuably differentiate very similar and reputable excipient suppliers, but a partner who is an expert in this area will be able to provide more insight – as well as offering other advice about analytical data that can contribute to additional business decisions!
By working collaboratively with a partner and keeping an open mind to suggestions around analytical testing, you’ll be able to obtain data that can help accelerate development, demonstrate the understanding needed for a robust regulatory submission, troubleshoot effectively, and even develop a better supply chain. Most importantly, analytical data and expertise give companies the opportunity to establish detailed material profiles that will last for a lifetime.
- C Moore, “Quality by Design – Lessons Learned and Challenges for International Harmonization”.Presentated at the International Conference on Drug Development, Austin, Texas, US, February 28, 2012.
- Grand View Research, “Pharmaceutical Analytical Testing Outsourcing Market Size, Share & Trends Analysis Report By Service (Bioanalytical, Stability Testing, Method Development & Validation), And Segment Forecasts, 2019 - 2026” (2019). Available at: https://bit.ly/33dXifg. Last accessed: October 10, 2019.
- Micromeritics, “The Definitive Guide to Porosity”. Available at bit.ly/2N6dzhm. Last accessed November 1, 2019.
- Micromeritics, “The Definitive Guide to Particle Sizing”. Available at bit.ly/2PDnBYW. Last accessed November 1, 2019.
- European Pharmaceutical Manufacturer, “Why powder testing is a powerful tool for healthcare” (2019). Available to view at: https://bit.ly/2WuNB9R. Last accessed: October 10, 2019.
- R.B.P Pinto et al., “Role of Particle Morphology on Tablet Dissolution Rates”, Pharmaceutical Technology, 43, 30-34 (2019).
General Manager at Particle Testing Authority, a Micromeritics company.



















