
Melt in the Mouth
Orally disintegrating tablets are winning patients over thanks to ease of use, but manufacture is unconventional, which raises a number of challenges and the need for careful consideration.
Though tablets are the most common dosage form, some people have an aversion to – or even fear of – swallowing them. In the mid-1990s, orally disintegrating or dissolving tablets (ODTs), began to appear on the market. Dissolving rapidly when placed on the tongue, the ODT is a convenient dosage form for many groups of people, particularly those likely to have swallowing difficulties, such as mental health, geriatric or pediatric patients. But they are also good for people who simply do not like taking tablets, for whatever reason.
ODTs typically have very good shelf life and do not require refrigeration, which simplifies transportation and storage. Additionally, as the drug is absorbed via the mucous membrane within the oral cavity, it avoids first pass metabolism in the liver and provides rapid onset of action. For that reason, ODTs are particularly popular for therapeutic categories where very quick relief is preferable, such as painkillers, treatments for gastrointestinal disturbances, and anti-allergy medications.
The ideal ODT should be both physically and chemically stable, and not too large – typically, about 500 mg is the maximum size. The tablet should disintegrate completely within the mouth in no more than 30 seconds, and should give no unpleasant sensations, whether that is an offensive taste, a gritty texture, or a burning sensation in the mouth or throat. To overcome these challenges, formulators generally opt for either a loosely compressed ODT or a lyophilized ODT. With each method, there are some specific considerations to bear in mind.
Loosely compressed
The loose compression process for manufacturing ODTs is not that dissimilar to producing traditional tablets. As well as the active, three main functional ingredients are required for a loosely compressed tablet to impart rapid disintegration: super-disintegrants, effervescent agents and soluble agents. Super-disintegrants, such as sodium starch glycolate, crospovidone or croscarmellose sodium, will either swell or wick up the liquid on contact with the saliva, disrupting the tablet’s structure and encouraging dispersion. The effervescent agent is commonly sodium bicarbonate in conjunction with an organic acid – normally citric or tartaric acid. Contact with saliva causes effervescence and, again, affects the structure of the tablet. Soluble agents, such as xylitol or mannitol, should be included to assist with tablet disintegration in the mouth.
Numerous other excipients can also be added to impart specific properties, such as sweeteners, colors and flavoring agents. Excipients that aid in the direct compression process are also beneficial in formulation development, such as fillers, lubricants and binders. However, some additional ingredients, though necessary in certain formulations, can impede disintegration; for example, high levels of lubricant.
The particle size must also be carefully considered; if it is too large the tablet may give a gritty and unpleasant mouthfeel as it disperses. The key processing parameter when manufacturing loosely compressed ODTs is the compression force. ODTs are compressed at much lower forces than traditional tablets. If the force used is low, it may improve the disintegration properties of the final tablet, but it is likely to result in an extremely friable tablet that may fall apart in transit or when handled. At the other end of the scale, if the compression force is too high, the tablet will be more robust, but disintegration could be affected. To that end, choosing the most appropriate compressible excipients to balance the strength and dissolution properties is extremely important.
Recent developments in loosely compressed ODTs include specifically designed highly compressible excipients. Excipient blends formulated to enhance disintegration are also available from a range of suppliers. Indeed, several providers offer blended excipients in a ready-to-use form for the creation of loosely compressed ODTs, including F-MELT from Fuji Chemical Industries Co., Ltd, which combines inorganic excipients and disintegrants with a carbohydrate complex. Another commercially available mixture, Ludiflash from BASF, is a mix of crospovidone, polyvinyl acetate and mannitol.
To manufacture a loosely compacted ODT, the usual method involves the drug active being blended with the excipient mixture and then moistened with a solvent (usually water or ethanol). This is then molded into a tablet via low compression. A step in which it is treated with heat or air should be carried out to remove excess solvent, and in some cases, promote a solid-state excipient phase transformation, which increases the hardness of the tablet.
Freeze drying
Lyophilized ODTs do not rely on super-disintegrants to provide rapid dispersion; instead, rapid disintegration results from the way in which they are manufactured and the formulation of excipients. For example, an ODT may use gelatin to form the overall tablet polymeric structure, in combination with mannitol, to increase robustness and attractiveness. Both ingredients dissolve readily in saliva, giving a quick-acting, melt-in-the-mouth experience for the patient. The active and excipients are all dissolved, or suspended if they are insoluble in water. The solution (or suspension) is then dosed into blister trays, before being frozen in a liquid nitrogen freeze channel. The blister trays are then lyophilized. Lyophilization involves the sublimation of ice crystals from within the formulation, leaving behind a network of air pockets within the tablet’s structure. The porous matrix of gelatin, mannitol and active ingredient that forms the ODT will be left behind.
The porous structure formed during lyophilization is key to achieving rapid disintegration. The highly porous nature of the tablets allows saliva to wick into the tablet and cause disintegration. As with a loosely compressed ODT, a range of other excipients can be incorporated to impart specific properties, including taste masking agents, sweeteners, flavors and pH modifiers. With careful formulation, the creation of a dosage form that is easy and pleasant to take should be possible. The most critical excipients are those that form the porous structure – the gelatin and mannitol. While the freeze drying process is under way, it is important to ensure that all of the mannitol remains crystalline in the finished dosage form, or there will be a risk that it will collapse on storage. Using the optimal conditions for freezing is crucial. If the tablets are frozen too quickly, then small ice crystals are likely to accumulate, which will affect the ODT’s porosity.
One of the key challenges when working with an ODT is taste masking. Many drugs have an unpleasant taste and taste masking is therefore necessary to obtain a palatable formulation. Taste masking can be achieved by three main principles: covering the unpleasant taste sensation with a pleasant one, preventing contact between the trigger molecule and a patient’s taste receptors, or by inhibiting the taste receptor response.
Traditionally, taste masking a lyophilized ODT was a challenge due to the resulting particle size formed by the coated particles leading to a larger tablet, but it is now possible to achieve taste masking on tablets up to around 400 mg by coating the outside of micronized API particles in a vessel that is equipped with an acoustic vibrator. These particles can be as small as 100 µm in diameter. Additionally, the process does not require solvent, leading to process improvements. As an alternative, the active ingredients can be held inside cyclodextrin molecules, which prevent them from touching the taste receptors on the tongue.
From jabs to tabs
Other recent developments include the ability to create stable oral formulations of vaccines and other protein and peptide drugs, traditionally administered by injection. Moving from an injectable dosage form to an ODT can improve patient compliance, especially for pediatric populations. Additionally, the creation of a stable room temperature dosage form provides cold-chain advantages, especially for developing countries. Some ODT platforms can also enable the sublingual or buccal delivery of biologics, such as peptides, proteins, allergens, and vaccines in an ODT formulation. There are no extreme pH exposure or proteases in the oral cavity and this route avoids the harsh environment of the gastrointestinal tract. For vaccines, this technology offers the potential to eliminate cold-chain storage, and can be beneficial for mass immunization programs and emergency response. Our platform uses a lyophilization process with low processing temperatures, and there are formulation options to optimize in-process stability such as through matrix component selection or pH adjustment. The dried product has low water activity to ensure long-term stability.
As an example of a marketed biologic ODT, Danish pharmaceutical company ALK-Abelló launched Grazax as a patient-friendly allergen immunotherapy for the treatment of grass pollen-induced allergic rhinitis. Patients previously had to make monthly visits to the clinic for their subcutaneous injections. Grazax is a tolerogenic vaccine, which increases resistance to allergens and can be disease modifying in patients.
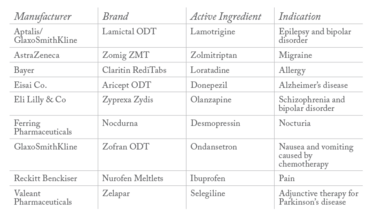
Table 1: Examples of approved ODTs
Melting innovation
The production processes for ODTs can be seen as costly compared with traditional tablets and capsules, but actually the difference is not so great – especially when considering the advantages of ODTs – ease of use, rapid onset of action, easy dosing properties and wide acceptance by patients. Perhaps the biggest drawback of ODTs right now is their limited ability to incorporate higher concentrations of active drug, but this is improving with research. New manufacturing techniques are also emerging. For example, a new type of ODT was introduced in 2015 based on 3D printing. Antiepileptic levetiracetam, under the brand name Spritam, is manufactured using Aprecia’s ZipDose technology. ZipDose “prints” multiple layers of drug powder, tightly packing them together into a porous, water-soluble matrix. The process creates a high dose tablet (up to 1000 mg) that dissolves instantly with just one sip of liquid, which breaks the bonds formed during the printing process. Taste masking technology can also be applied.
Another alternative to ODT is thin film strips – a delivery technology that can be used for both systemic and local action via several routes of administration including oral, buccal, sublingual, and even ocular and transdermal routes. The technology is considered easy to swallow, self-administrable, and fast dissolving. However, application of film strips is somewhat limited as the maximum dose that can be formulated for delivery is in the 20–50 mg range.
ODTs are still relatively new – the FDA approved its first ODT in December 1996 – but with manufacturing costs coming down and increasing calls for more patient centric medicines, we can expect more ODTs in the future. Perhaps one day the industry will soon be able to create ODTs for immunogenic vaccines, helping to reduce reliance on injectables – and representing a significant advance for patients.
Elizabeth Hickman is Strategic Marketing Director, Oral Drug Delivery, at Catalent Pharma Solutions, NJ, USA.
Serves as Chief Executive Officer at AustinPx, bringing over two decades of expertise in the biotech and pharmaceutical sectors, formerly serving in leadership positions for West Pharmaceutical Services, Catalent, and Pii. Elizabeth holds a BA in Microbiology from The University of Texas at Austin and an MBA in Marketing from San Diego State University.



















