Printing medicines for express delivery through the cheek
For the past decades, the buccal epithelium (inner side of the cheek) has been explored as a potential administration route for drugs. This route is particularly favored for the administration of biologics because of its systemic circulation drainage and low enzymatic content, as well as the fact that it bypasses the gastrointestinal tract.
Buccal films have emerged recently as one of the most suitable dosage forms for the buccal administration route because they are patient-friendly, small, and can incorporate an adequate dose amount. Previous research in our group has been directed towards developing a nanostructured film for the buccal administration of insulin (an example of a biologic) as a potential medicine for diabetes (1). Although these nanostructured films show great potential – and we continue to research to further develop this technology – some limitations have been identified. Therapies that benefit from biologics usually have very strict requirements in dose and frequency that vary from patient to patient. To address this and other limitations of our first generation of films, we are currently exploring the use of inkjet printing to accurately dose strict amounts on films for buccal delivery of biologics.
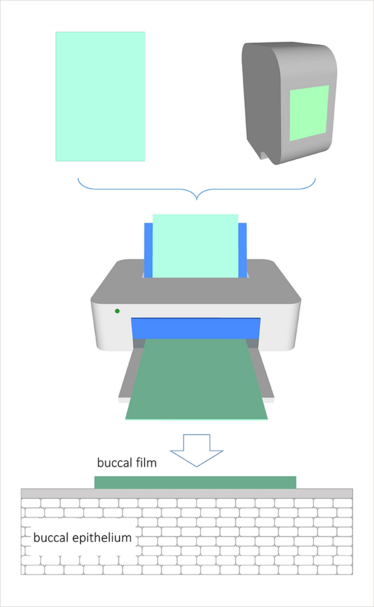
Inkjet printing has attracted a lot of attention from the drug delivery field. Most of the research to date has been geared towards printing small molecule drugs, but very little has been reported on the printing of biologics. Our investigation presented at the 2015 CRS Annual Meeting in Edinburgh, Scotland (2), is our first step in the process of printing biologic drug models (Figure 1). We have found that lysozyme and ribonuclease can be printed in a dose-proportional fashion with remaining activities (indicative of their stability) in a wide range, depending on the conditions studied. More importantly, we have shown that the use of a permeation enhancer in the ink formulation can have a damaging effect on the biologic’s activity. Although all these results are encouraging and show that printing of biologics as ink solutions is feasible, we currently rely on an optimization stage; that is, we expect to increase the bioactivity of the drug molecule after the printing process.
Either 2D or 3D, printing of drug molecules is an emerging field and we expect it to only keep growing. While most of the research efforts are put into the printing of small molecule drugs, biologics represent a large and growing part of pharmaceutical R&D, and finding alternative routes for their administration is a pressing issue in the field. We foresee and hope that the future will bring advanced printing technologies for patient-tailored delivery systems of biologics.
- J. O Morales, et al., “Films Loaded with Insulin-Coated Nanoparticles (ICNP) as Potential Platforms for Peptide Buccal Delivery” Colloids and Surfaces B: Biointerfaces, 122, 38–45 (2014).
- J. O. Morales, et al., “The Use of Inkjet Printing for Dosing Biomacromolecular Actives in Drug Delivery Systems” In 2015 CRS Annual Meeting and Exposition, Edinburgh, Scotland, 2015.



















