Rising DAMPP
A promising new process – dropwise additive manufacturing of pharmaceutical products (DAMPP) – brings medicine production down to size.
With the introduction of Process Analytical Technology and Quality by Design, the pharmaceutical industry has been looking towards innovative manufacturing methods to consistently and accurately deliver quality products (1). Traditionally, pharmaceutical manufacturing processes have been large-scale batch processes that employ little by way of automation or process control, and so waste product, time, and money, when the product is off-specification. Smaller-scale processes and continuous processes allow more flexibility, advanced process control and real-time process management. At the Engineering Research Center for Structured Organic Particulate Systems (ERC-SOPS), we have developed a number of these mini-manufacturing systems. Here, we describe one such promising approach – dropwise additive manufacturing of pharmaceutical products (DAMPP) (2–5).
What is DAMPP? Put simply, DAMPP uses drop-on-demand technology to deposit active pharmaceutical ingredients (API) directly onto substrates. Drop-on-demand technology, a concept widely used in inkjet printing, has become popular in other applications in recent years, particularly with the rise of 3D printing. Drop-on-demand technology is now used to deposit everything from nanoparticles to polymers to molten metals, and has begun to be used in biological applications, such as the production of cell cultures (6, 7), tissue or organs (8) or pharmaceutical products. For example, GlaxoSmithKline have used their Liquid Dispensing Technology system in large-scale production to deposit API dissolved in liquid solvents onto placebo tablets (9). Drop-on-demand is generating a lot of interest: it is flexible and easy to operate; there are very few unit operations; it is easy to changeover between different materials; and drop size can be precisely adjusted to control the amount of material deposited. However, drop-on-demand systems must be carefully controlled and automated to ensure precision and on-specification product (10). On a smaller scale and with careful selection of process parameters and printing materials, the use of drop-on-demand technology in DAMPP allows for precise control over the final drug product and leads the way to several innovations in pharmaceutical manufacturing.
DAMPP can be used for small-scale production of dosage forms in the pharmacy, as opposed to mass-manufacturing products in a plant – ideal for a high-potency, low-dose API, which requires greater precision per patient than that achievable by large-scale manufacturing. DAMPP also leads the way to individualized medicine: many researchers have proposed using clinical data, genetic analysis, and/or patient plasma samples to tailor the dosage amount for each patient, and maximize the therapeutic benefit (11). Whichever way the optimum dosage is predicted, the approach requires a flexible manufacturing method, which has not been available so far. DAMPP is perfectly suited to such a role, especially for APIs that exhibit high inter-patient variability. DAMPP can even be used to control the morphology of the final drug product. By depositing API mixed with polymers, it is possible to produce specific crystalline forms or amorphous forms of drug substance, which can help create drug products with higher dissolution rates or higher bioavailability that are still stable during processing and storage. Finally, DAMPP could be used to layer different drug products on one composite dosage form, which would be useful for patients who take several medications at once, such as patients undergoing chemotherapy, as well as allowing dissolution control, enteric coating, taste masking, and abuse resistance.
So far, our work on the DAMPP system has involved developing the prototype along with the accompanying real-time process management system, which includes automation, process control and fault detection methods. We have used DAMPP to deposit two types of formulations (solvent- and melt-based), both of which have been used to control the final morphology of the dosage form.
The DAMPP Process
The DAMPP system, shown in Figure 1, consists of a drop-on-demand positive displacement pump system, XY staging, and accompanying control and communication systems.

Figure 1. The DAMPP system, with drop-on-demand positive displacement pump system, XY staging, and accompanying control and communication systems.
The formulated printing material starts in a reservoir and is pumped out of a Teflon or stainless steel nozzle positioned over the substrate. The size of the drop formed can be broadly selected with the nozzle size, and fine-tuned with settings on the positive displacement pump. The drop is deposited onto the substrate mounted on the XY staging, which moves the substrate under the nozzle, so the drops can be deposited in a grid pattern, as seen in Figure 2.

Figure 2. Drops deposited onto a dosage form in a grid pattern.
Real-time process management strategy
Real-time process management for DAMPP consists of automation, regulatory control, monitoring, and exceptional events management (4). Each of these aspects is needed to control drop dynamics and ensure a quality, on-specification product.
Automation
Each element of the DAMPP process is synchronously executed by means of a LabVIEW program, which allows the user to input various process parameters, start the process, and then monitor the process with real-time temperature readings and images. The program follows the flow in Figure 3.
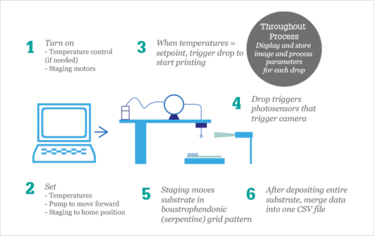
Figure 3. Process flow of DAMPP.
Regulatory Control
The temperature is maintained for the entire process, and control is implemented along various points in the DAMPP process, as seen in Figure 4.
Controlling the temperature at the material reservoir, pump and tubing/nozzle allows material properties to be maintained, which is particularly important for melt-based solutions that need to be liquefied above ambient temperature. Controlling the temperature of the product on the XY staging underneath the substrate using Peltier devices allows control over the final product, both in terms of morphology and to solidify the dosage, by either evaporating the solvent or solidifying the molten polymers.
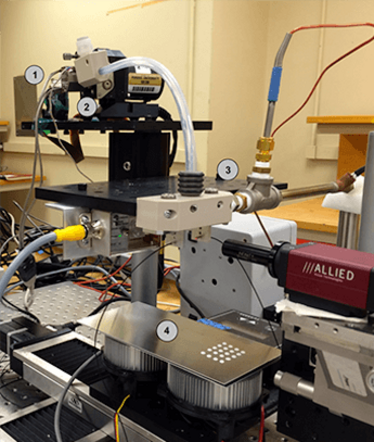
Figure 4. Points of temperature control in DAMPP.
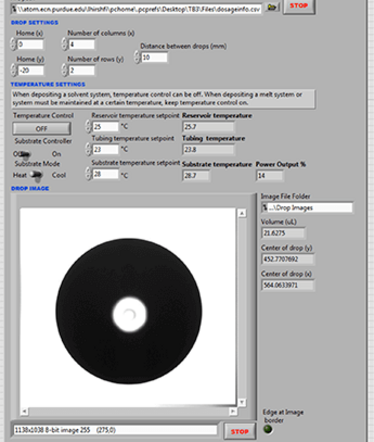
Figure 5. The user interface of the DAMPP program.
Monitoring
Feedback control, in which a correction to the process is applied based on a deviation of the current measurement from the desired setpoint, is not necessarily applicable to drop dynamics; given the rapidity of drop formation, measured deviations cannot correct the current drop but can be used to correct the following drops. For example, if a drop is found to be smaller than desired (and thus depositing less API than expected) there can only be changes made to subsequent drops to make up for this error. Therefore, the implementation of monitoring strategies on drop-on-demand systems is a necessity to ensure product quality.
The DAMPP system contains a camera with accompanying photosensors and a backlight, which is triggered when a drop ejects from the nozzle. Each image throughout the process is displayed in real time, as shown in a screenshot of the user interface in Figure 5.
The image is analyzed to calculate the volume of the drop, so if you know the concentration of API in the printing material, you also know the amount of API in that drop and the cumulative amount of API deposited on the substrate. This calculation, along with the drop location, drop images, temperature readings of various points of the process, and other data, is saved automatically and archived for easy reference.
Exceptional Events Management
Imaging each drop also allows for detection of various faults in the process. Selection of printing material and process should lead to a quality product, but there are faults that can occur outside of the expected random deviations within the process. Faults, or exceptional events, can arise due to deviations in drop dynamics, malfunctions of the printing system, or failures in the monitoring or control equipment. Besides calculating the volume for each drop, the program also calculates the center of each drop to detect deviations in drop trajectory, which can be caused by clogging or fouling at the nozzle tip. The program also ensures that the drop number and image number match up; if they do not match, a drop fell without being triggered, or a drop image was not captured even though a drop was triggered. In these cases, the operator is alerted, and the program halts.
DAMPP products
The DAMPP prototype has thus far been used to demonstrate production of two types of dosage forms: solvent-based and melt-based. Solvent-based formulations consist of a solvent, API and sometimes a polymer. Melt-based formulations consist of a polymer and API that are liquefied at the mixture’s melting point. The use of polymers in the formulation not only improves printability of the fluid and adhesion of the drops to the substrate, but allows for control over the final product morphology. Therefore, DAMPP can be used to produce stable amorphous or specific crystalline forms. The formulation type and polymers used can be selected based on the API’s crystallization behavior and the desired morphology.
The DAMPP prototype has proven to be effective in printing solvent-based formulations, pictured in Figure 2, consisting of naproxen (chosen for demonstration for its rapid crystallization properties), polyvinylpyrrolidone (PVP), and ethanol (2). We printed drops using both a 30:70 and a 70:30 Naproxen:PVP formulation, with 1 gram of solid dissolved in 10mL of the ethanol solvent. Using varying drop sizes, we were able to produce completely amorphous forms (with the 30:70 formulation) or semi-crystalline forms (with the 70:30 formulation) varying from 33 percent to 77 percent crystalline.
For melt-based formulation, we can use a low-melting-point polymer (or a lipid or surfactant) such as polyethylene glycol (PEG 3350) or Pluronic F38, in which the drug dissolves once the polymer becomes molten (3). We printed molten drops containing 15 percent naproxen and 85 percent polymer on inert tablets, as shown in Figure 6. Depending on the polymer used, we observed differences in drug dissolution behavior, and thus differences in the bioavailability of the drug. Although naproxen is crystalline in the presence of PEG and Pluronic, different deposit morphologies can be achieved by controlling the drop solidification process (5).

Figure 6. Deposition of melt-based formulation on inert tablets.
To summarize, DAMPP is a mini-manufacturing process that deposits API directly onto a substrate, leading the way for small-scale production in pharmacies to create individualized dosage forms, layered pharmaceutical products, and shelf-stable amorphous or semi-crystalline dosage forms. With appropriate selection of process parameters and drug formulation, DAMPP gives precise and accurate control over dosage amount, morphology, phase, composition, and release profile, all while monitoring and controlling the process and product in real time.
As the landscape of pharmaceutical manufacturing changes, there is a need for such innovative, automated, and flexible processes with advanced process control, to enable personalized medicine.
- T. C. Tyson, “Pharmaceutical Manufacturing Innovation”, J. Pharm. Innov. 2(1–2), 37–37 (2007).
- L. Hirshfield et al., “Dropwise Additive Manufacturing of Pharmaceutical Products for Solvent-Based Dosage Forms”, J. Pharm. Sci. 103(2), 496–506 (2014).
- E. Icten et al., “Dropwise Additive Manufacturing of Pharmaceutical Products for Melt-Based Dosage Forms”, J. Pharm. Sci. 104(5), 1641–1649 (2015).
- L. Hirshfield L et al., “Real-Time Process Management Strategy for Dropwise Additive Manufacturing of Pharmaceutical Products”, J. Pharm. Innov. (2015). doi:10.1007/s12247–015-9218-5.
- E. Icten, Z. K. Nagy and G. V.Reklaitis, “Supervisory Control of a Drop on Demand Mini-Manufacturing System for Pharmaceuticals”, 24th European Symposium on Computer-Aided Process Engineering, Computer-Aided Chemical Engineering, 535–540. DOI:10.1016/B978-0-444-63456-6.50090-9 (2014).
- E. Roth, “Inkjet Printing for High-Throughput Cell Patterning”, Biomaterials 25(17), 3707–3715 (2004).
- N. Sanjana and S. Fuller, “A Fast Flexible Ink-Jet Printing Method for Patterning Dissociated Neurons in Culture”, J. Neurosci. Methods. 136(2), 151–163 (2004).
- V. Mironov et al., “Bioprinting: a Beginning”, Tissue Eng. 12(4), 631–634 (2006).
- IChemE press release, “GlaxoSmithKline Wins Top Prize at IChemE awards” (2010). www.icheme.org/media_centre/news/2012/glaxosmithkline wins top prize at icheme awards.aspx#.UmqWEPk0ASN.
- Q. Xu and O. A. Basaran, “Computational Analysis of Drop-On-Demand Drop Formation”, Phys. Fluids 19(10), 12 (2007).
- Blau, G.E, S. Orcun, J.M. Lainez, G.V. Reklaitis, A. Suvannasankha, C. Fausel, and E.J. Anaissie, “Validation of a Novel Approach for Dose Individualization in Pharmacotherapy Using Gabapentin in a Proof of Principles Study”, Pharmacotherapy, 33, 727-735 (2013).
Laura J. Hirshfield obtained her Ph.D. in chemical engineering at Purdue University, completing her dissertation on the development of and the automation and control of DAMPP. Upon graduating, she switched her focus to engineering education research, aiming to study the teaching and assessment of industry-relevant non-technical skills like open-ended problem solving and innovation. She is currently a postdoctoral researcher with a joint appointment in Olin College of Engineering and the University of Michigan’s Center for Research on Learning and Teaching in Engineering and will begin a faculty appointment at Rowan University in fall of 2016.
Elçin Içten was born and raised in Istanbul, Turkey. While studying chemical engineering at Bogazici University, she participated in a study abroad program at Purdue University. After graduating from undergraduate, she came back to Purdue and started graduate school in the School of Chemical Engineering. Currently, she is a Ph.D. candidate working in the manufacture of personalized medicine.
Arun Giridhar is a research scientist in pharmaceutical manufacturing at the Center for Structured Organic Particulate Systems at Purdue University. He works on continuous manufacturing, advanced process control, and the precision printing process for personalized medicine.



















