The Long Road to EXCiPACT
If I knew six years ago just how much of my time EXCiPACT would take up, would I have even started? A good question! But, actually, I have no regrets; the story of EXCiPACT and its impact, even today, on the pharmaceutical industry makes every second worthwhile.

One distant day in the last century…
I was annoyed. I had travelled halfway across Europe to meet with an industrial customer to promote our new metalworking products but the majority of the meeting had been taken up by discussing poor product quality for our existing business. Sadly, it wasn’t the first time I’d heard about these problems, so I decided to go down to the manufacturing plant to find out what was happening. The answers were not comforting: while there was control over the process, there was insufficient understanding of the chemistry involved, so when abnormal situations were encountered they did not know what to do. As a chemist I knew I could help.
And so began my journey into quality assurance (QA) and, indirectly, down the long road to the establishment of EXCiPACT.
If you work in pharma manufacturing, you will almost certainly have heard about EXCiPACT, a global, third-party certification scheme for pharmaceutical excipient suppliers. Here, I tell the story behind the standards.
The trauma of audits
When I graduated from the University of Bristol with a BSc and PhD in organometallic chemistry, I had no idea where my career would take me. I took a research position at BP, before joining Croda in 1987, where I have had a great career ever since. In my first Croda role, I provided technical support to sales teams, but I found my vocation in QA. By drawing on my experience in chemical research I was able to help the manufacturing team increase product quality and manufacturing efficiency. Croda customers were much happier and this freed up time in our meetings to discuss new products and ideas. An opportunity presented itself to work in R&D and although I loved the customer interaction, the chance to go back to my roots in the laboratory was too strong to resist. But this was just a stepping stone, and a new role in quality allowed me to use all my skills and knowledge across the business. All too soon another customer-centric problem came to my attention.
I was completely perplexed by the variety of outcomes from customer audits. The majority of customers were perfectly happy with our processes and quality standards, but a few would audit the exact same product, made in the exact same way, and then be very unhappy and worried. Clearly, some auditors were assessing us with very different standards, and those differences seemed to arise chiefly from the intended use of the product. At that time, I knew of no uniform standards or best practice for excipient suppliers. I was pointed in the direction of the UK Pharmaceutical Quality Group (PQG), and they provided me with some very helpful guidance. Rashly, I commented at the launch event that if they ever needed help with a revision of that guide I’d be happy to help. Well, that comment was the beginning of my involvement in setting standards for excipients.
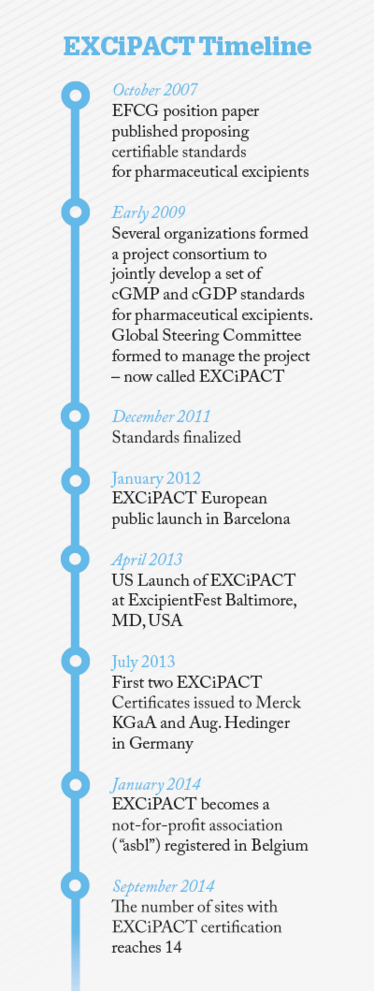
Working with the PQG in the UK in the late 1990s, we designed a new GMP standard for pharmaceutical excipients – PS 9100. At its core was a tiered approach, based on an initial risk assessment, with increasingly strict standards of quality and purity depending on the use of the excipient and other factors. These allowed different standards to be applied for topical creams, oral products and injected medicines. When PS 9100 came out in 2002, it was leading-edge – even revolutionary, I might say. It took the rest of industry and the authorities 12 years to catch up. PS 9100 already contained many of the building blocks of what would eventually become EXCiPACT.
At the time, PS 9100 was controversial in using a risk-based approach to determine the degree of GMP that was required. It attracted a lot of criticism and was not widely adopted, not least because it was a UK standard, but I always knew the approach was fundamentally correct in terms of assuring patient safety. A little later the International Pharmaceutical Excipients Council (IPEC) –PQG GMP guidance for excipients was published in 2006. The IPEC-PQG excipients GMP Guide used many of the same concepts as PS 9100 but in a less formal manner. It was more a document of its time and as a result it has been very well accepted by regulators and industry worldwide. Many companies adopted it and used it to audit their suppliers, but as a guidance document, rather than a standard, it was not ideally suited for this purpose.
Guidance is primarily educational; it sets out the principles to follow and provides an explanation of them in various scenarios. Guidance is a “how to do” approach, which is great to educate and inform on best practices but causes problems with audits. An example: a favored word in guidance is “where appropriate”. Well for me that begs the question – who decides what is appropriate? Is it the auditor who comes to assess your compliance? Or the excipient supplier? The lack of clarity is not helpful. By contrast, a standard is a “what to do” document – if you want to know how, then read the guidance. A highly respected member of the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) was always amazed that PS 9100 was so short: “I can’t believe you have reduced GMP to so few words”. Less is more, as they say.
Tragedy triggers change
While most manufacturers were making progress in quality, it became clear that the industry as a whole was not doing enough. Heartbreakingly, in the late 2000s, the industry saw several serious instances where GMP failure, or failure to secure the supply chain, led to patient deaths. One of the most widely reported cases was the adulteration of heparin with counterfeit ingredients from China, which caused more than 80 deaths and 700 serious adverse reactions. There were also a number of instances worldwide of glycerin, used in cough syrups and baby teething medicine, being contaminated with toxic ethylene glycol, with hundreds of fatalities. The tragedy is that these deaths were avoidable – implementation of very simple GMPs would have prevented them all.
The string of tragedies understandably alarmed the authorities, who needed to tighten up regulation and increase control of all ingredients – not just the finished dosage form and active pharmaceutical ingredients, but also the excipients and other ingredients. Tighter regulation generally means more audits. I went to my sales team and asked how many pharmaceutical customers we were working with. The number was large. Assuming each customer audits every three years, we were looking at several audits every week at every site. We couldn’t keep up with that, and on the other side of the fence pharmaceutical companies were making a similar calculation and realizing they would need an army of auditors.
It wasn’t just the number of audits causing concern. If regulators started to inspect excipient suppliers, in Europe at least, they could only audit them to Part I or Part II GMP. These standards are designed for the world of medicinal products, and while the principles of ensuring product purity are the same in excipient manufacture, the actual detail is fundamentally different. The classic example is an auditor used to working with medicinal products coming to a chemical plant and citing absent hairnets as a noncompliance. The plant management then point out that everything is encased in stainless steel reactors and pipes, running at several hundred degrees centigrade and extreme pressure – if anyone came into close enough contact with the product to drop a hair in it, contamination would be the least of their problems! In this environment, protective clothing is worn to protect the workers, not the product. That’s not to say the product should not be protected from contamination, but a hairnet is not likely to be much use in that situation. The risks to product quality stem from the technology used to make the excipient. A one size fits all rule is not going to be effective in all circumstances, especially given the complexity of excipients, their varied sources and methods of manufacture.
For many excipient suppliers, like Croda, pharmaceutical excipients are only a part of the business. If the GMP or auditing requirements became so onerous that it was financially unrewarding to supply this market, suppliers would simply stop providing excipients. The impact of withdrawal of suppliers from the pharmaceutical industry on the availability of modern medicines would be huge. It was clear we needed to raise the bar for excipients, but I also knew that it had to be done at a pace that was viable for the whole industry. PS 9100 had taught me that lesson.
EXCiPACT is born
Excipient suppliers and pharmaceutical manufacturers all agreed that something must be done to stem the rising tide of audits. The obvious solution was third-party certification – an approach that had been successfully applied before in industry – just look at the way ISO 9001 has been adopted worldwide. But the weaknesses of ISO 9001 were well documented and understood. So in July 2008, there was a meeting of a number of organizations representing suppliers, distributors and pharmaceutical companies. The meeting is still sharp in my memory, since it did not get off to as good a start as I expected. I suspect I wasn’t alone in this!
We could all see that there was a problem, and we were all very passionate about protecting patient safety. But everyone had different ideas about what should be done. Many of us had never met before and several times during that first meeting I found myself thinking, “I can’t work with these people!”
But despite the stormy start, gradually we found some common ground. Once we had the basis of that initial agreement, everything quickly lined up. EXCiPACT was born!
The energy of that meeting really carried us forward over the next few months as we formed working teams for GMP, good distribution practice, defining standards for auditors, and certifying bodies.
It took nearly three years to define, design, develop and consult on those standards. We consulted widely, with regulators and other stakeholders. This was the most crucial part of the process and we built on the success of the IPEC document in this respect – the wide-ranging consultation and visible review of those comments has been instrumental in getting acceptance of EXCiPACT and building quality into the whole scheme. There were many other trials and tribulations during that time, and several points when I nearly regretted taking on the project. It was clear that it was not going to be a short haul process. But equally I and the EXCiPACT team were convinced we had a really good solution to the problem of audits, and like many quality professionals, I don’t like to give up.
In these early stages, we tragically lost one of our key members, Arnulf Heubner, who died suddenly. His leadership was very important in forming EXCiPACT and we felt – and still feel – his loss greatly.

Writing and preparing standards was only one part of the solution. Having been in QA for some time now, I thought I was aware of the business systems and processes needed to run and make a success of an organization. But I had been quite insulated from money matters and my education was about to get a rude awakening as we brought EXCiPACT to its launch.
At the start of 2012, we formally launched the scheme in Barcelona at the IPEC Europe Annual Meeting. Suppliers pay what we believe to be a modest fee of €5500 every three years for certification, revenue that EXCiPACT uses to provide direct oversight of both certifying bodies and auditors. We are working very hard to make sure standards are kept high. EXCiPACT already has its very own QA manager – and now a Treasurer too, because all that oversight and QA requires financing. The irony of learning about the theory of businesses through QA and the reality of financial matters is not lost on me!
EXCiPACT delivers more than a certificate – an audit report is provided to the customer by the supplier, so they can evaluate the quality systems in place and perform their own risk assessment. The supplier can then decide whether they need to carry out their own audit, or whether the EXCiPACT certification is sufficient.
Importantly, at the launch, we were joined by representatives from the UK’s MHRA and the US FDA, who were very positive and made it clear that EXCiPACT matched their requirements for demonstrating GMP. Though regulators have the authority, in reality they do not have the resources to routinely inspect excipient suppliers, so they view the scheme as a welcome development in helping to assure the quality and purity of excipients.
Impact of EXCiPACT
Initial reactions to the scheme have been very positive. Currently, there are 14 certificates held by different suppliers. Our initial goal is to get 20–30 organizations certified, and expand the scheme in the US and Asia. Companies, both suppliers and users of excipients, have reported that they have significantly cut down on the number of customer audits. We believe passionately that we have a good product – one the industry can use, that is cost effective and which doesn’t compromise on quality. But ultimately the customers – the excipient users – will decide if it is a success or not.
Of course, people do have concerns. One thing that suppliers have remarked on is the length and thoroughness of the audit. At least one supplier has told us that they have never had a customer audit as difficult as the EXCiPACT audit. But I take that as a compliment – we need to set a thorough standard. We need pharma companies to know that they cannot do an audit better than one by EXCiPACT. That said, anyone who thinks EXCiPACT is going to stop all customer audits is dreaming; that is never going to happen. However, I do believe it will certainly halt the increase in audits that many manufacturers have been facing.
The road ahead
When flying over to Paris for a conference, most of us don’t typically think about all the hard work and effort that has gone into getting that plane into the air (and down again). It’s the same with medicines. Patients have an expectation that a medicine is going to work, and that it’s not going to harm them. But it takes a coordinated effort from manufacturers, suppliers, regulators and many others to make sure that is the case. We all use excipients – you, me, our families – and we all want those products to work and be safe.
When I look back to where we were over 25 years ago at the beginning of my time with Croda and where we are today with the quality and purity of our products, I can hardly believe the progress we have made. It’s absolutely astonishing. I see the next 20 years as a continuation of that. I don’t think we can or should stop now, when there is still so much more we can do to ensure patient safety. EXCiPACT is certainly part of that journey, but it’s not the final destination.
The last six years have not been easy, that’s for sure. But I’m proud to have been able to work with a large number of equally committed people, who have given up their time as volunteers, to make EXCiPACT happen. In ten years’ time, I’d like to look back and see that I started something that has helped make medicines safer.
At university, Iain Moore spent time deciphering nuclear magnetic resonance spectra to determine the atomic structure of organometallic products that he had synthesized. It was an inspiring and captivating area, but didn’t satisfy his need to apply the knowledge to real world problems. “A career in industry – predominantly with the oleochemical supplier Croda – put all my problem solving skills to the test.” Combining these skills with the desire to help people do better led him naturally to quality assurance, and then to working internationally on the definition of best practice standards for pharmaceutical excipients and now bio-based products. “Along the way, I like to think I’ve helped solve one or two real world problems.”



















