Better, Faster, CRISPR
Pharma companies look to new genetic screening technology to enhance drug discovery
Clustered regularly interspaced short palindromic repeats (better known as CRISPR) has caused quite a stir in the scientific research community. Essentially, it allows precise genetic changes to be made and its potential as a drug discovery tool has not gone unnoticed by pharma companies. Novartis signed off a CRISPR deal at the end of January and now AstraZeneca has announced its own CRISPR research project; the company will collaborate with four research partners (Wellcome Trust Sanger Institute, the Innovative Genomics Initiative, Thermo Fisher Scientific and the Broad Institute/Whitehead Institute) to use CRISPR to identify and validate new drug targets in preclinical models in certain therapeutic areas. It’s an open innovation project, which means the findings will be shared between the research partners and published in peer-reviewed journals.
We spoke with Kosuke Yusa, a member of the Sanger Institute Faculty, to find out why we’re likely to see CRISPR in more pharma discovery labs in the future.
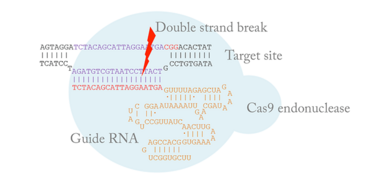
Mechanism of CRISPR
How does CRISPR work and why is it so exciting?
It has been known since the early 1990s that DNA double-strand breaks (DBSs) in mammalian cells are recombinogenic and that it is possible to edit the genome using the endogenous DSB repair machinery. However, there was no technology at the time that allowed us to induce DSBs at a specific site in the genome. The CRISPR-Cas system is a new technology consisting of two components: a guide RNA and a Cas9 endonuclease. They form a ribonucleoprotein complex and induce a DSB to the genomic site determined by the guide RNA. The system is incredibly simple compared to other gene-editing technologies. We can generate a new reagent for the CRISPR-Cas9 system in less than a week at the cost of only a few dollars.
How exactly can it help drug discovery?
RNAi screens have been a powerful means to drug discovery, but they require a large initial investment and considerable running costs; typically, results from a limited number of test subjects (usually just one cell line) are used to identify new drug targets. CRISPR screens are cheaper and more scalable. Multiple samples can be tested, which will allow us to comprehensively identify drug targets. Pharmaceutical companies can also use CRISPR to generate reporter systems to measure the efficacy of candidate drugs.
How did the collaboration with AstraZeneca get started?
When we published our paper describing CRIPSR-based genetic screens in Nature Biotechnology (1), I received an email from my current collaborator in AstraZeneca mentioning another potential collaboration using our technology for drug discovery. Subsequently, we had several meetings and discussed potential projects. We are now in the process of recruiting two postdoctoral scientists who will work on the project.
How successful has CRISPR been so far?
Success stories to date are centred around technology development. One particular example is in vivo genome editing. A paper published in Nature Biotechnology last year (2) showed that a mutation was reversed into wildtype and mice suffering from a gene defect were completely cured. The paper showed the potential of CRISPR as a therapeutic agent.
What’s the future of CRISPR?
As a tool for genome-wide mutagenesis, CRISPR is more than perfect as it is, in my opinion. As a tool for genome editing, there have been a number of techniques developed. There will be some refinement to increase overall efficiency of genome editing, but the current form of the technology is efficient enough for most applications. In the next couple of years, CRISPR will become a routine technique, like PCR, and there will be many new discoveries.
- H. Koike-Yusa et al., “Genome-Wide Recessive Genetic Screening In Mammalian Cells With A Lentiviral Crispr-Guide RNA Library,” Nature Biotechnology 32, 267–273 (2014).
- H. Yin et al., “Genome Editing With Cas9 In Adult Mice Corrects A Disease Mutation And Phenotype”, Nature Biotechnology 32, 551-553 (2014).

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















