Bringing Vaccines into the 21st Century
Medicine manufacturing has benefited from countless advances in technology over the last few decades, and yet many vaccines are still being produced with decade-old processes. Change is never easy, but is falling behind really an option?
sponsored by GE Healthcare

Mats Lundgren has an intense interest in the field of vaccines, with an academic and professional background to match. Dr. Lundgren’s passion is understandable; vaccines have helped conquer numerous healthcare challenges and no doubt have a great deal to offer in the future. Despite their value, many vaccines are still being manufactured using legacy technology such as eggs or animal tissues. Today, Lundgren works as Customer Applications Director at GE Healthcare, where he helps companies with implementing modern processes. The end goal? More efficient production and higher vaccine quality.
What was your route into GE Healthcare?
I’ve worked for several biotech companies over the years, but the reason I joined GE Healthcare in 2008 was because I wanted to be more applications-focused and to work more on the technologies used in the biomanufacture of monoclonal antibodies and vaccines. Vaccines are a really interesting area for me. Not only do they have a major impact on health worldwide (it is thanks to vaccines that we were able to eradicate smallpox) but they are also interesting from a technology point of view. We have seen lots of advances in this area, particularly in terms of single-use technology. At GE, I support our customers with application knowledge, such as how to use innovative products and how to implement new processes.
What are the global trends in the vaccine industry?
Consolidation is one big trend right now. It’s being seen across the developed pharma and biopharma industries because of cost pressures and the need to be more efficient. In the vaccines area, we’ve seen major deals such as GlaxoSmithKline’s acquisition of Novartis’ global vaccines business, which took place earlier this year. Large vaccine manufacturers based in Europe and North America are also seeing increased competition from developing markets. More and more companies, mainly in Asia but also in Latin America, are setting up their own domestic vaccine production. In some cases, this is for their own market, but many companies are starting to export. For example, The Serum Institute of India is a huge exporter of vaccines to UNICEF.
Importantly, I think we’re also seeing a greater appreciation of the value of vaccines. In the 70s to 90s, vaccines were to some extent considered low-profit products, but now decades of research is starting to come to fruition with the development of more advanced vaccines. Some vaccines could have the potential to even treat disease, such as cancer vaccines. I believe these advances have renewed interest in the vaccine field.
What are the main problems with traditional vaccine production methods?
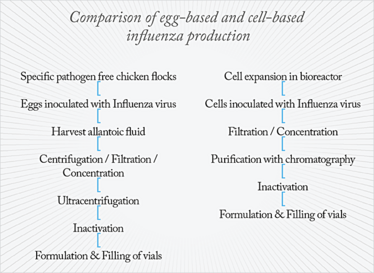
Some vaccines on the market are produced using egg-based processes and technologies that were developed decades ago. Egg-based vaccine manufacturing is a lengthy process and companies have to predict demand ahead of time. Production can’t be accelerated or ramped up in case of a pandemic, and sometimes there may even be situations where eggs cannot be secured in the correct numbers, such as during an avian flu outbreak. Other processes may include a lot of manual handling (for example, with open flasks during expansion of adherent cells), which can be a quality risk. Moreover, the demand for human resources makes production costs high. And it’s not only the technology that is behind the times; the industry still uses a lot of animal-derived raw materials, which can carry the risk of contamination.
These drawbacks are being increasingly recognized by the vaccine industry, particularly in light of increased competition in the field. Vaccine manufacture needs to be faster and more efficient – subsequently, companies are starting to look at how they can bring processes into the 21st century. If you’re wondering why it’s taken so long to come to this realization, you need to consider that vaccines haven’t traditionally turned big profits, which didn’t match the fact that modernization requires investment. In addition, vaccines tend to be used in healthy individuals (and many children) and must not give rise to unwanted side effects. Thus, there was a mentality that if the old processes work then why should they be changed? And what if changing a process brought about a new side effect? Costly clinical trials might be required to show that the new processes indeed can produce safe and efficacious vaccines. In my experience, updating processes improves product quality, especially as that usually means using the most modern systems, which have been specifically designed to improve manufacturing. Change, of course, always involves an expense, but this can be balanced by a better process economy (and lower production costs) in the long term.
What changes are being made?
We are seeing a shift away from egg-based to cell-based production, which is a very well-defined process. Changes are also being seen in technology; instead of centrifuges, you can use chromatography to purify vaccines; instead of stainless steel bioreactors that are difficult to clean, you can use single-use bioreactors; instead of growing cells on the surface of (many) flasks you can grow the cells on microcarriers, which are tiny beads inside a stirred bioreactor. There are also newer cell culture products available that help to more efficiently propagate the viruses and bacteria, as well as analytical tools that can control and track what is happening throughout the whole vaccine production process. The key benefits of all of these new technologies is that they are faster, more efficient and take up less space. Most new technologies have also been designed to accommodate the industry’s need for more flexible manufacture by being modular and disposable.
How do attitudes to new technology vary among companies?
Overall, I believe that most companies are really keen to use the latest systems available to them, but at the same time they are also cautious. Many established companies have been using the same processes for 50 years or so. Their facilities are well established and often built around these old plumbed-in processes so it’s challenging to accommodate changes – both from an infrastructure point of view and a regulatory point of view given that they are working with long-approved products. But this doesn’t mean that updating is impossible. I don’t think it’s very useful to tamper with an established process just because of cost, but if it benefits vaccine quality or purity then the change will be appreciated by regulators because it will result in a better, safer product overall. A complete retrofit of a plant may be difficult but smaller steps can be taken; for example, getting rid of tissue culture flasks and moving to disposable bioreactors. This change can easily be justified because of the quality benefits.
The big opportunity for change for established manufacturers comes when they are developing a process for a new vaccine, expanding production or building a new plant. There is an opportunity here to employ modern technologies, gaining the benefits right from the outset. I see a lot of companies – even large, experienced ones – that try to work with processes that were originally developed for lab-scale work rather than commercial manufacturing. Scale up in this instance can be a frustrating experience. Working with modular, scalable technologies right at the beginning saves a lot of time.
Companies new to vaccine manufacturing are perhaps more able to implement the latest technologies because they are designing their processes and plants from scratch, so there is a real opportunity to get a competitive edge on established companies by employing modern, efficient manufacturing technologies. Some of these new manufacturers are located in areas where the regulatory framework might not be as well developed as perhaps Europe and the US. However, these countries are catching up very rapidly and, as mentioned earlier, companies in developing markets are keen to export and will be looking at technologies that can facilitate the consistent production of products in line with global quality requirements. Not all companies are aware of the complexities of establishing a new vaccine plant, particularly one that aims to export. And this isn’t just a problem in developing countries – any company anywhere in the world can encounter production difficulties and trouble with scale up, but this is where we come in with our advice and support. It’s not just about selling technology – it’s important to offer support and knowledge too. And this increases trust between the vendor and customer – and means that our products are used in the best possible way.
How can companies overcome the challenges of change?
Knowledge is crucial. First of all, you need to have a solid understanding of your processes and product to understand where the opportunities for change and an increase in product quality and production efficiency lie. Next, you need a good grounding in the latest production equipment and single-use systems so that you can see how these will fit into your processes – or how they can be used to create a new process from scratch. Finally, you need regulatory knowledge so that you can understand current requirements.
At GE, we’ve tried to raise awareness of the problems facing vaccine manufacture and of the benefits of new technology. We speak with our customers frequently to understand their problems, we speak at conferences and we are also starting to work with industry organizations, such as DCVMN – the Developing Countries Vaccine Manufacturers Network. This is a powerful organization where manufacturers in developing countries can share their knowledge of new production technologies, as well as regulatory and quality aspects.
What are the real risks of being left behind?
A big part of my role is to visit companies and to talk about the different technologies and how they can be implemented in different processes. I always propose changes that will impact the final vaccine product in a positive way. Companies that don’t embrace the potential benefits of modernization could become obsolete. More and more companies are keen to enter the industry and this growing competition means you can easily become outdated. That may sound a bit dramatic, and I don’t expect to see companies immediately dropping out of the market, but to secure a long-term future, I think you need to examine the benefits of updating your production processes. It’s very tempting in the pharma business to stay with the same old technology that you know and trust, but it’s an attitude that can come back to bite you sooner or later. We are firmly in the 21st century. Do we really want to be producing life-saving products with legacy systems?



















