
Capturing Value in Changing Times
The increasing complexity of biologics raises significant manufacturing challenges – not least regarding cost control and downstream purification. Thermo Fisher Scientific’s answer? To boost capture efficiency with a scalable, broadly applicable platform technology called CaptureSelect (TM).
sponsored by Thermo Fisher Scientific
Innovative biotherapeutics promise to more fully meet patient needs, but only if manufactured at appropriate cost and quality. Unfortunately, standard purification processes tend to be incompatible with advanced biologics. This mismatch adds time and expense to biotherapeutics development, as manufacturers often must develop a new process using inefficient capture systems. Fortunately, there is a better way. Imagine adopting an affinity resin system that could be customized for nearly any biologic – and rapidly scaled up to cGMP manufacture. We asked the Thermo Fisher Scientific’s experts to tell us more about CaptureSelect affinity resins.
Laurens Sierkstra (Business Segment Leader, CaptureSelect Affinity Products) has been involved in CaptureSelect technology from the beginning and has almost 25 years’ experience with the technology.
Pim Hermans (Manager, CaptureSelect Ligand Discovery) also worked on CaptureSelect technology during its genesis and today continues to liaise closely with clients to develop the ideal affinity resins for their needs.
What changes have you seen in biologics manufacturing?
Laurens Sierkstra: Back in 2003, everybody focused on standard monoclonal antibodies purified with Protein A. In fact, the original business plan for the CaptureSelect technology began its life as a direct competitor for Protein A. But we immediately found that our customers wanted to process molecules for which Protein A was unsuitable, so we developed purification products that customers needed – resins for recombinant proteins, non-standard antibodies, gene therapy vectors, and other innovative biologics.
Pim Hermans: Discovering that customers were moving to biologics incompatible with Protein A was a real eye-opener for us – and this shift in the biologics landscape continues today. Fifteen years ago, customers might have wanted to purify Factor VIII; now they want to purify exosomes, viral vectors, or hard-to-process antibody fragments, while avoiding co-purification of light chains. Clinical pipelines reflect this evolution. Back then, over 90 percent of biologics entering the clinic were monoclonals; today, 25–30 percent comprise entities such as viral vectors, cell therapies, bispecific proteins, antibody fragments, and Fc fusion proteins.
Have other aspects – for example, timelines – also become more challenging?
LS: Well, manufacturers have always wanted to get to the clinic as fast as possible! Fortunately, the CaptureSelect platform has time advantages as it can purify virtually any biologic without needing to develop a whole process from scratch. Just as manufacturers use Protein A and polishing to purify standard monoclonal antibodies, CaptureSelect is a standardized platform for purifying biologics that Protein A cannot accommodate. Thus, CaptureSelect accelerates processes by reducing downstream complexity.
PH: Notably, the technology isn’t just for non-antibody products – it also meets antibody purification needs that Protein A cannot address. For example, we can direct ligand specificity to precise FAb or Fc domains, thus enriching for advantageous properties.
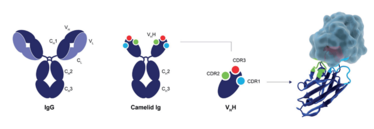
CaptureSelect technology is based on the variable domain of Camelid heavy-chain only antibodies (single domain or VHH fragment). In contrast to conventional IgG molecules, camelid antibodies are devoid of light chains but they maintain the same level of specificity. VHH fragments are exceptionally small antigen binding fragments (~15kD) which allows binding to alternative epitopes, leading to a unique affinity profile. Compared to standard antibodies, these fragments are very robust and can withstand the harsh conditions used during chromatography.
What makes CaptureSelect unique?
LS: Firstly, the technology works through antibody-based selectivity, so we can develop an affinity resin for virtually any biologic – indeed, we have never failed to make a purification system for a proteinaceous molecule. Others have tried to make antibody-based affinity resins, but conventional antibodies are somewhat unstable, expensive to produce, and difficult to upscale. CaptureSelect, however, uses single domains from antibody heavy chains: these are robust and compatible with large-scale manufacture. And that’s why they are ideal for affinity resins intended for biotherapeutics manufacture.
Secondly, CaptureSelect is highly efficient. Remember, biologics must be manufactured at an acceptable cost-of-goods, and this requires high yield. Reaching the clinic quickly with an inefficient process only results in a cost-of-goods disadvantage compared with a manufacturer who has a more efficient process. Our capture step provides increased yields, partly because of its intrinsic efficiency and partly because we can design our resins to preferentially select active (rather than inactive) forms of the biologic. Thus, CaptureSelect provides manufacturers not only with high yield but also with a high proportion of functionality.
Thirdly, CaptureSelect reduces the number of purification steps, which saves time and cost. And finally, fewer columns, in turn, reduces the clean-room footprint, and increases efficiency of clean-room utilization. Together, these four attributes give manufacturers a valuable cost-of-goods advantage.
How do you help manufacturers who are struggling with biologics purification?
LS: Our modus operandi is highly collaborative and customized. Put simply, we begin by understanding the client’s problem, and then we develop a program to solve that specific issue. Often – due to CaptureSelect’s high selectivity – we can suggest solutions that manufacturers have not even considered.
PH: Usually, manufacturers assess available purification products and then design a process that fits those products. But we do it the other way around; we work with the client to identify the ideal process, and then we make a resin that fits the ideal. Conventional purification technologies can’t provide customized resins that perfectly match the needs of a given client.
How else do you differ from other providers of purification technology?
LS: Firstly, we are a one-stop shop: we can take clients all the way from initial concept to a fully developed affinity resin compatible with GMP manufacture. We have the ability to both produce a ligand of interest and make a scalable, GMP-compliant affinity resin. And that requires excellent infrastructure and expertise. We were fortunate in that we had the right assets from the very beginning. Many companies with good ligand identification technologies have failed because they could not turn ligands into products that can be manufactured at appropriate quality criteria and scale – finding something that binds a particular molecule is the easy part! But with CaptureSelect, we can guarantee development of a GMP-compliant affinity resin within about ten months, scalable from ~1 mL to ~200 liters as necessary. In brief, manufacturers need certainty regarding scale, price and timescale, and we provide all three.
If we only offered ligand discovery technology, and not the ability to make scalable, GMP-compliant affinity resins, we would have to license the affinity ligands to clients. Instead, our model is to make and sell affinity resins for clinical trials and cGMP manufacturing.
How are you positioned to meet future challenges?
LS: Biologics will continue to become more complex; advanced Fc fusions, fusion proteins, new gene therapy vectors (such as exosomes or red blood cells), or allogeneic cell therapies are key trends. But we too will evolve; we are always adapting the CaptureSelect technology to address more complex products by working closely with customers.
PH: We ensure that we keep track of market developments. Years ago, we realized that adeno-associated virus vectors would become important, and developed products for that niche. Today, we are doing likewise for exosome technology. The goal is always to give customers excellent downstream processing tools – while staying ahead of the technology curve.



















