Is Biopharma Ready to Say Goodbye to Batches?
Very few biologics are made using continuous processing equipment like bioreactors. Clearly, the biopharmaceutical industry has a long way to go before it’s ready to embrace (potentially) superior manufacturing models. Why?
Ronald A. Rader, Eric S. Langer |
Biopharmaceuticals are perhaps the most complex of all high-tech products, yet the most fundamental aspects of their manufacture have remained unchanged for decades. Most other modern industries have adopted continuous manufacturing (1), exemplified by the automobile assembly lines Henry Ford introduced over 100 years ago, petroleum refineries and newer steel mills, which run non-stop. In contrast, nearly all biopharmaceutical product manufacturing still involves batch processes.
Mature industries have long figured out how to adopt continuous manufacturing because it is more efficient and cost-effective. Continuous processing can, when done well, manufacture the same product using much smaller-scale equipment and fewer people. For example, consider a steel mill that heats, melts, refines ore and pours the metal into molds, with each component process done on a very large scale as a single batch – it might process 100 tons at a time with one batch per week, producing 5,000 tons/year. Now compare that with a mill with the same manufacturing capacity, but operating continuously – if two tons are processed every two hours, it would produce 8,500 tons/year. Moreover, the in-process materials need not be moved into and out of storage, meaning that labor needs are lower and more evenly spread.
The same holds true for biologics manufacturing, where a continuous process at smaller scale not only saves resources, space and labor, but also allows better process monitoring and control – in an industry where this aspect is vitally important. And if something does go wrong with continuous processing, only the affected material need be collected and discarded, rather than an entire batch.
Old/new technology
Continuous bioprocessing (CPB) is not new, and a few commercial products have been manufactured using perfusion technologies for several decades. For example, adherent fiber-based bioreactors have been in common use for monoclonal antibody (mAb) manufacturing using fused-cell hybridomas since the 1980s, before recombinant mAb manufacture became dominant.
But these technologies took a back seat to batch bioprocessing for a variety of reasons. One is that continuous processing can require more sophisticated operations. In our annual report on biomanufacturing, we asked 238 bioprocessing professionals about issues concerning perfusion versus batch-fed processes. Of the 19 areas evaluated, the Top 3, where a majority of respondents reported perfusion as presenting more concerns (versus fed-batch) included:
- Process operational complexity: Complexity introduces elements of risk that in a biopharma situation can create very costly, sometimes disastrous operator errors. The simpler, the better, in most cases.
- Process development control challenges: Optimizing bioprocessing is an ongoing project, and if a batch process at small-scale is tweaked, a clear outcome can be observed and extrapolated to improvements at larger scale. In continuous bioprocessing, controlling all the parameters is sometimes more challenging.
- Contamination risks: Risks of contamination of a process is a major industry concern. Worries about how those risks may affect a CBP facility put this high up on the list of potential concerns.
Though many of the perceived problems with perfusion have already been improved, there appears to be continued resistance to adoption of new processes. Perceptions and attitudes regarding perfusion will likely change for the better in coming years, as more bioprocessing professionals develop hands-on experience with perfusion operation and its advantages.
In classic batch method manufacturing, antibodies are commonly manufactured using bioreactors and other vessels holding more than 10,000 liters, with process preparation (cleaning, sterilization) and processing taking three weeks or more. In contrast, using a 500-liter perfusion bioreactor (where spent, product-containing culture media is continuously removed at same rate that fresh media is added) and continuous chromatography equipment for purification, the same or likely more product can be produced in the same overall time – as there is far less of the down-time associated with batch processing.
Comparative studies at largest mammalian scales, such as blockbuster mAb manufacture in fixed stainless steel facilities, continue to show improved cost–benefit ratio using fed-batch, with perfusion more cost-effective at up to 500 kg/year capacity. However, building a batch facility could cost more than $120 million, while the much smaller CBP facility could cost $25–35 million. Operating expenses will be comparable or lower for the continuous facility. This is because a continuous operating facility will tend to be be smaller, and operated with higher productivity (more production over time), compared with a batch bioprocessing facility making the same product.
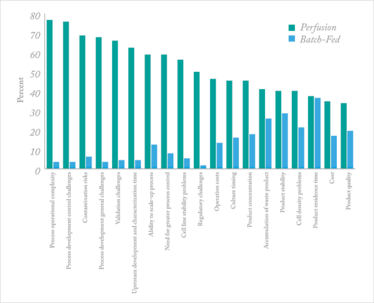
Figure 1. Comparison of perfusion versus batch-fed bioprocessing concerns (Source: 12th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, www.bioplanassociates.com/12th)
State of play
Adoption of basic changes in industrial manufacturing methods takes decades – it took over 50 years for continuous steel casting to dominate that industry. And the bioprocessing industry, with its regulated operations, is even more conservative. For example, it has taken well over 10 years for single-use systems using disposable plastic equipment to be introduced at small-scale operations, and it will require at least another decade before these processes are substantially adopted for large commercial-scale biopharmaceutical manufacturing. In the biopharmaceutical industry, adoption of CBP has so far been restricted to a few unit processes being implemented at a minority of facilities. By far, the CBP currently most recognized involves smaller perfusion bioreactors operating continuously for much longer periods of time. Currently, about a dozen, less than 10 percent, of biopharmaceuticals are manufactured using perfusion bioreactors.
The issues restricting adoption of CBP involve the ‘complexity’ concerns and prejudices noted above, but there are also some significant engineering, design, and integration problems to be overcome. Other major challenges include the need to cost-effectively implement CBP at commercial scales while meeting rigorous regulatory requirements for product quality, predictability and control. The difficulties are surmountable using current technology, but there is a lack of practical know-how and concerns over the availability of cost-effective and specialized equipment usable at larger scales.
CBP has also not been adopted for purification and other downstream processing operations, especially at commercial scale. There are many variations of CBP for downstream operations in development, but they have not yet made the big-time. Some, such as simulated moving bed (SMB) and periodic countercurrent (PCC) chromatography are projected to be 20–30 percent less costly compared with current methods. But even that isn’t enough to motivate the industry to quickly adopt an alternative.
Regulatory agencies, particularly the EMA and FDA, are often cited as holding back CBP adoption. But these agencies have generally been very open to CBP. From a regulatory perspective (as with the engineering aspects), the difficulty revolves around overcoming industry inertia that favors the incumbent process, simply because it’s easier to get a clunky, more expensive manufacturing operation through the onerous regulatory process than it is to introduce a robust novel bioprocess. So although CBP can make better products, at smaller-scale, and offer superior quality compared with conventional fed-batch manufacturing, adoption will take time. Aspects as simple as how to define a ‘batch’ have long been cited as hurdles. And although regulatory agencies are increasingly familiar and competent in these areas, few biomanufacturers want to be the guinea pigs in a regulatory test case, when they can simply select a tried (tired) and trusted manufacturing strategy.
An eye on the horizon
Today, existing bioprocesses are typically upgraded to CBP in isolated process steps; for example, fed-batch bioreactors are adapted for perfusion with the addition of devices such as the ATF (alternating tangential flow) system perfusion pumps from Repligen. However, for new drugs, process lines and facilities, CBP will use more broadly integrated technologies that encompass multiple process steps.
Process control and automation is a very important component – and great advantage – of smaller-scale continuous processing. Moreover, statistical methods, such as Process Analytical Technology and Quality by Design (QbD), intended improve and document processes are more amenable to application to continuous versus intermittent batch processes.
By essentially simplifying process and product quality control, CBP can reduce out-of-spec excursions and intra-lot variability.
It’s not all plain sailing. Early adopters of CBP for marketed drugs may encounter issues that reflect the immaturity of the technology in our field; equipment is not always specifically designed for the increased rigors of long-term continuous use, and depending on the application, stainless steel may be preferable to single-use equipment. Indeed, for some very large-scale biopharmaceutical manufacturing, it may end up being more cost-effective to use large dedicated fixed stainless steel systems operating in batch mode. A big question is: is there a future for large-scale biopharmaceutical manufacturing anyway? (see “Precision Biomanufacturing” 0715-504).
Some biomanufacturers have become leaders in CPB implementation, including Genzyme and Bayer; both have a great deal of experience in using perfusion for commercial product manufacture. Amgen recently opened an integrated $200 million CBP manufacturing facility in Singapore. Hemispherx Biopharma makes a cell cultured immune modulator that has allowed an 80 percent reduction in related staff. Other companies using perfusion-based CBP include Roche, Johnson & Johnson, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Samsung, Biogen, Novartis and BioMarin, among others. As technologies advance, more suppliers will develop relevant equipment, and more end-users will implement their own approaches. As the industry gains experience and regulators become more comfortable, widespread CBP adoption seems inevitable.
- BioPlan Associates, "12th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production", April 2015, Rockville, MD www.bioplanassociates.com/12th
- S. S. Farid, B. Thompson and A. Davidson, “Continuous Bioprocessing: The real thing this time?”, mAbs 6, 6, 1357–1361 (2014).
- S. S. Farid, J. Pollock and S. V. Ho, "Evaluating the Economic and Operational Feasibility of Continuous Processes for Monoclonal Antibodies", In: G. Subramanian (Ed.) Continuous Processing in Pharmaceutical Manufacturing. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany. Ch 17, 433–455 (2014).
- J. Pollock et al., "Optimising the Design and Operation of Semi-Continuous Affinity Chromatography for Clinical and Commercial Manufacture", Journal of Chromatography A, 1284, 17–27 (2013).
- J. Pollock, S. V. Ho and S. S. Farid, "Fed-Batch and Perfusion Culture Processes: Operational, Economic and Environmental Feasibility Under Uncertainty", Biotechnology & Bioengineering, 110(1) 206–219 (2013).



















