Making Media Better Than Average
Cell culture media developers used to focus on maximizing protein yield. But yields are yesterday’s problem...
sponsored by GE Healthcare
Cell culture media developers used to focus on maximizing protein yield. But yields are yesterday’s problem, and some might ask if it’s worth continuing to tweak culture media – isn’t what we have today good enough? No, because your cell culture medium can improve biomanufacturing output above and beyond mere titer.
Peggy Lio is Global Leader of Cell Culture and Process Sciences at GE Healthcare. She originally started her career in big pharma working on Intron A, one of the first biotherapeutics to receive FDA approval. After seeing the bioprocessing industry evolve from the early days through to its current complexity, Peggy is curious to see what the next developments will be. Here, she discusses how she applies her experience from both sides of the fence – as an end user and as a supplier – to help improve cell culture process performance.

What is your role at GE Healthcare?
I lead a team of senior process scientists and our remit is to help people with their cell culture process development issues. And this is a great opportunity for me because I am able to use my experience from large pharma to help customers with the same problems that I myself have faced and overcome. So we collaborate with customers and offer solutions to issues they may have and make recommendations for how the process can be improved based on our experience. There’s also a lot of connectivity to experts in related bioprocessing areas – both upstream and downstream. For example, cell culture media is intimately linked to mixing technology to ensure the right preparation for large-scale use. Or if a customer has an issue at the bioreactor stage, I can link our process development expertise to the know-how we have in bioreactors. At the end of the day, it is all about providing holistic, integrated solutions that provide performance and efficiency gains.
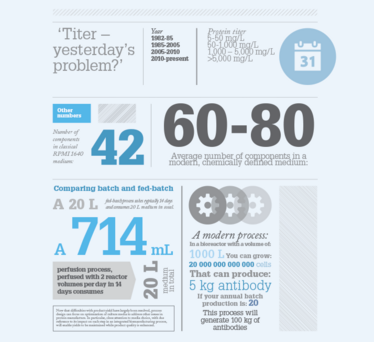
What general trends are you seeing in cell culture media?
About 10 to 15 years ago, the focus was on optimizing monoclonal antibody titers in CHO processes to support the manufacturing of blockbuster biotherapeutics. The main driver was to increase titers to decrease COGS. Years of effort went into that and now there are many excellent cell culture media available that can support the production of high titers of antibodies – 3-5 g/liter or more – from CHO cell lines. So today, titer is no longer the main issue and the current focus is instead on product quality and reducing variability. Biosimilars have influenced this shift because developing a biosimilar is not as straightforward as developing a small molecule generic. If you think about a biosimilar compared to the innovator product, starting from a cell all the way to a final therapeutic, nothing in the production process will be exactly the same; the cell line is going to be different, your process is going to be different and your medium is going to be different. The medium that was originally used for the innovator process is probably a decade or more old (and quite likely proprietary to the innovator) and may not include some of the recent advances in cell culture media design. For example, the original medium may contain hydrolysates. Hydrolysates have been associated with quality issues so the industry is now aiming to move away from them entirely. We often work with both innovators and biosimilar developers on how we can improve media formulations to take advantage of all the lessons learned in the manufacture of large molecule biotherapeutics.
Looking at other product areas, the trends are a little different. Vaccines, for instance, are still evolving; many companies still use legacy processes with different cell types and media, including non-chemically defined, serum-containing media. While it’s difficult (and costly) to change traditional vaccine production processes, I think we need more modern media designs, especially for new vaccines. Historically, vaccines were to some extent seen as a commodity, but this is changing rapidly now and the a lot more attention. A recent example is the Ebola breakout and the need for an Ebola vaccine.
Another area receiving increased attention in the industry is continuous processing – and this means that cell culture media need to be specifically evaluated and optimized for perfusion processes. This is a great example of why it is good to have a breadth of experience; it’s not just about understanding media, but understanding the whole process and how the medium fits into it.
Does this mean that culture media should not be seen as a stand-alone product?
Right – cell culture media should be considered in the context of the whole process. For example, some cells are more shear-sensitive than others, so you may need to increase the amount of shear-protectant in the medium if you are operating under high-shear conditions. However this may have implications downstream so a change in the medium formulation might warrant additional changes elsewhere. Another example is when you have a cell line that is prone to clumping; we can often fix clumping by modulating the concentration of specific components in the medium. But again, this change needs to be evaluated from a holistic process perspective so that you don’t surprise your downstream colleagues at a later stage. Therefore, you should never consider cell culture media in isolation.
What issues do your clients often ask you to solve?
A common issue is the trade-off between product yield and product quality. There is definitely a balance that needs to be struck, but addressing product quality comes first, followed by finding ways to improve titer without sacrificing your quality requirements. It isn’t always an easy process to address product quality, but we have found that if post-translational processing isn’t right, there are things we can tweak to get you the desired variants and the glycoprofiles. And it’s easier to adjust processes and media upfront than to remove unwanted protein variants at a later stage! We recommend tweaking your parameters and media at the very beginning, when you are selecting cell clones that will move into process development for biomanufacturing.
Another time when clients may encounter problems is at scale-up – after a final cell clone has been selected. During clone selection and at the beginning of process development, you use ready-made, liquid media in small volumes. But when you scale-up, it is often easier to utilize powder media for a variety of reasons; longer expiry period, lower shipping costs and less demand on warehousing, to name a few. So as you scale-up, you use powder, it’s shipped as a powder, and reconstituted at the site of use. But then the question becomes, is the liquid I used in the lab the same as the liquid being used at the site where my biotherapeutic is manufactured? The quality of the water used to rehydrate the medium may be different from site to site. Similarly, mixing with water at the liter scale on the lab bench is not the same as rehydration at large-scale. There can be issues of time, pH, sterile transfer methods, storage conditions, and stability. Reconstitution at manufacturing-scale is not straightforward; even such an apparently simple step can change significantly with scale-up.
How do companies choose the right cell culture media?
We advise customers to consider the cell culture medium as early as possible – right when they start thinking about cell line development. This is especially important given the tight time constraints associated with biotherapeutic development. So when you are choosing your clone, you should also be thinking about the cell culture medium and its impact on your complete process. Critical media attributes need to be thought through very early on to ensure that you find the right solution, or provide the right guidance to your supplier to create a custom solution. Do you want the medium to be chemically-defined and animal origin free? Do you want an off-the-shelf product, your own formulation, or one customized to your specific cell line and process? An off-the-shelf product will be more readily available, and there are multiple high-performing media out there, but a customized medium will be optimized for your own cell line, so you should expect better results.
Do you think that the next-generation processes will trend towards using ready-made media or customized formulations?
I believe that if you work closely with a quality, experienced media supplier then you can probably get similar results with off-the-shelf media as you would with customized media, in a much shorter time. Although granted, there are times when customization is required to achieve performance, process or efficiency goals. It’s all about understanding your needs and how the cell culture medium fits into them. And that in turn suggests the ideal supplier profile; customers should choose a company which is flexible, accessible and communicative, and that has relevant expertise. This expertise should enable you to have a choice of off-the-shelf media developed with the latest insights, as well as the know-how to quickly customize a solution if the situation requires it. The supplier should also resemble the client in terms of exposure to the whole bioprocess. Cell culture media is not a stand-alone product, and customers need a supplier that understands both the media and the process as a whole, and how the chosen medium fits into the bigger picture. In my opinion, many suppliers are similar when it comes to their access to manufacturing facilities and the quality of raw materials they use. The big difference is in their understanding of the entire process, which affects the services and products they can offer – and ultimately this will have an impact on the final quality of the cell culture medium that is delivered.



















