
mRNA is revolutionary – and it’s most probably here to stay. We speak with two experts from Sartorius – Integrated Solutions Manager Ganesh Kumar (GK) and Global mRNA Process Technology Manager Nargisse El Hajjami (NEH) – to ask their advice on the next generation processes and facilities that can help you master mRNA manufacture.
What are the biggest trends in mRNA manufacturing?
NEH: One could argue that mRNA manufacturing is, in itself, a trend! During the pandemic, our society witnessed just how effective, fast, and flexible an mRNA platform could be for vaccine production for an infectious disease. More and more biopharma customers now want their own mRNA manufacturing capacities, although the specific trends vary depending on what modalities and applications customers are pursuing. Without a doubt, many challenges remain in optimizing nucleic acid constructs, delivery systems, manufacturing processes, stability, and efficiency for mRNA products, and we see a big focus on applying innovation at different levels to support the rapid growth of the field.
One of the largest missing puzzle pieces today is a standard process template or platform for producing mRNA. The field is still in its infancy and we still don’t have that much data around existing technologies’ performance, process parameters, and yield control. In addition to this, the existing variability in mRNA constructs with various sizes and properties leads to variability in process performance and steps, which makes the standardization of a platform even more complicated.
We are also still missing stable and efficient solutions for mRNA delivery because currently there are still challenges in handling formulated nanoparticles at very low temperatures and assuring efficient delivery of mRNA into specific sites of the body. In this area too, we see a lot of work exploring innovative encapsulation methods and next generation delivery systems to assure safe and effective mRNA delivery to targeted sites.
Process design is another key challenge. There is an entire intellectual property landscape growing around process steps and the technologies used. A process can be the strength of a company, because with the same template you can potentially produce any mRNA sequence to produce any protein target and trigger different applications using the same process flow with minor process adaptation, and by simply changing the sequence content of your DNA templates.
GK: We are also seeing efforts to simplify facility design and reduce running costs by – to give a few examples – creating fully closed processes with an optimized single use (SU) setup, modular systems in a ballroom concept, and implementing in/at-line process analytical technologies for the measurement of critical process parameters (CPPs) and data analytics for prescriptive control.
In time, we may see the industry segment itself into a set of manufacturing platforms specialized for different varieties of mRNA constructs, different scales of production, and so on.
Why is the variability of mRNA design such an issue?
GK: First-generation mRNA processes were typically linear and used traditional mRNA, but there are numerous other types of viable constructs that are being explored, such as self-amplifying and trans-amplifying RNA, which could have a positive impact on cost of goods, drive process miniaturization, and require less doses (almost or greater than a log reduction in some cases) to treat patients.
NEH: The issue with variability is multi- levelled. First, we could be talking about mRNA variability in terms of sequence construct or type as mentioned previously. Specifying the type of mRNA – for example modified, self-amplifying, non-replicating, or circular – is important because different sizes, forms, and properties of mRNA demand the adaptation of process steps and used technologies. It’s not only the efficient purification of large size mRNAs that can be difficult – sterile filtration can also be near-impossible. One would need to set up alternative solutions, assure sterility, and meet the relevant regulatory requirements. Second, we could be talking about mRNA variability in terms of DNA template origin – for instance DNA template from E. coli compared to synthetic DNA – because different origins will produce different levels of contaminants. All of this can impact process design, potentially adding more steps to setup. More process steps will demand more space, more testing and validation, and inflict higher costs. The lesson here is that choosing the right strategy for mRNA with the end in mind is crucial. The fact that so many options are now in play only underlines this.
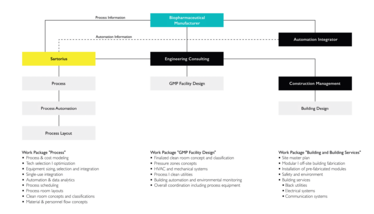
Figure 1: Sartorius Conceptual Design Setup for a state-of-the art SU facility
What considerations should factor into the design of new mRNA facilities?
NEH: The manufacturing process should be at the core of the facility design. You must ensure that you are implementing a process flow that is robust, efficient, fast, and cost-efficient – and that your facility is designed to support this process while allowing enough flexibility to quickly and easily adapt to changes and growth.
It is important to consider innovative technologies and next generation solutions for the different aspects of mRNA manufacturing while designing your mRNA facility, all to better support the need for speed.
GK: Some of the key questions that must be considered are scale, single product versus multi-product, current titres versus future state titres, localized versus centralized manufacturing, and in-house versus outsourced pDNA manufacture.
Though RNA-based modalities are relatively new, biopharmaceutical manufacturers can rely on the available standardized industry approaches and tools to help get started. For example, at Sartorius, we have a standardized conceptual design package that has been successfully applied in process and facility design for mRNA manufacturing. As outlined in Figure 1, we actively work with our customers to first model, optimize, and define the process with the associated technologies. After this, the equipment, SU concept, scheduling, automation strategy, and preliminary process layouts with personnel/material flows can be generated based on the process and its risk assessment (1). Through active collaboration the biopharmaceutical manufacturer, engineering, consulting, and construction companies, the outputs from the process package can be successfully incorporated into the GMP facility design and building packages to rapidly build the SU facility with modular/ prefabricated clean rooms, as required.
With such approaches, we successfully partner with biopharmaceutical developers across the globe to share our expertise and help them accelerate the design and implementation of their manufacturing strategy/facility.
NEH: To summarize, it is crucial to select the right manufacturing strategy and supply partners that can support you at different levels. You’ll need the right expertise and experts to help develop your process, optimize your manufacturing, design your facility, and select suppliers that will help you accelerate your journey.
How does Sartorius support mRNA developers?
GK: We offer customers solutions across upstream and downstream workflows for the manufacture of plasmid DNA.
For in vitro transcription and mRNA purification, we have a scalable bioreactor portfolio that offers a high degree of monitoring and control, as well as a scalable downstream toolbox based on convective monolithic chromatography media and flat-sheets/hollow-fibers for TFF applications. These allow us to accommodate different mRNA constructs using a platform purification approach.
Last but not least, we also offer services related to development and optimization of the process development and manufacturing workflows. For example, we work with our manufacturers to logically develop and optimize pDNA or mRNA purification platforms through Cornerstone Process Solutions. When dealing specifically with manufacturing workflow, we have conceptual design (as previously discussed) and value chain services that help our customers choose the right process, technology, Facility design, SU, and automation strategy to help them move towards a ‘smart’ modular facility.
NEH: mRNA storage and shipping are important aspects of mRNA manufacturing that require specific attention given the instability and sensitivity of mRNA products to handling, shear, and temperature upon lipo nano particle (LNP) formulation. For formulation and storage, we provide solutions covering development from the lab to large-scale manufacuring. On one hand, we provide a formulation and filling process development mRNA package to support mRNA developers on screening and identifying CPPs and critical quality attributes, and setting up their design space. They can then speed up their LNP development with a high throughput screening, tangential flow filtration, and controlled freeze/thaw system (2). On the other hand, we also offer a late- stage storage and shipping mRNA package that helps ensure stability during LNP storage and shipment, with a fully scalable freeze/thaw system and adapted storage solutions.
- Sartorius, “Bioprocess Consulting & Engineering” (Sartorius, 2022). Available at https://bit.ly/3pMmsPi
- Sartorius, “Lab-Scale Benchtop Freezing” (Sartorius, 2022). Available at: https://bit.ly/sr-freeze



















