Next Generation Bioprocessing and the Implications for Viral Safety
With many companies embracing the move to next generation bioprocessing, it is important that they do not forget to re-examine their approach to viral safety.
Kathy Remington, Michael Phillips |
sponsored by MilliporeSigma
Biomanufacturers are heralding next generation bioprocessing as a way to improve efficiency and productivity, reduce plant footprint and operating costs while maintaining the highest quality standards for the therapies being produced. Compared to traditional batch processing, this new manufacturing paradigm may include higher concentration fluids, higher mass loading of individual unit operations, longer duration processes and connected or continuous processes.
However, maximizing the benefits of this new approach to manufacturing requires consideration of the entire process from a holistic perspective. For example, process intensification using perfusion methodologies results in high density cell cultures that maximize protein productivity in relatively small bioreactors. While this can compress upstream timelines and increase protein yields per unit volume from the bioreactor, downstream operations may struggle to keep pace with higher titers from improved upstream operations.
It is clear that from a process development perspective, we need to consider the implications of efficiency improvements in a single operation in the context of the overall process. Overlaid on this, we may need to rethink how these changes might impact viral safety and how we assess the clearance capabilities of the individual operations.
Viral safety considerations with intensified processing
Higher concentration process intermediates, higher mass loadings on individual operations, longer duration processing, and connected or continuous processing all have the potential to impact viral safety.
- Higher concentration processing.
High protein concentrations could impact virus inactivation – either through changing the buffering conditions for low pH virus inactivation or potentially interfering with viral inactivation using detergents. In the latter case, as long as the concentration of detergent or solvent/detergent is maintained, the higher protein concentration is less likely to impact inactivation.
Highly concentrated loads may impact the performance of chromatography and filtration steps. Whether the chromatography step is run in bind and elute or flow-through mode, and regardless of the type of resin or membrane, the higher concentration of process intermediate, and potentially impurities, could influence the efficiency of the chromatographic separation. Viral clearance across the step may also be impacted through non-specific interactions of virus with the high concentration intermediate and the chromatographic resin, which may result in more virus binding to the intermediate or resin and consequently lower viral clearance. Similarly, higher concentration intermediates may impact virus filtration, necessitating increased use of prefilters to remove protein aggregates, or additional filtration membrane area. To confirm viral safety targets are met across downstream unit operations, viral clearance studies should be performed with higher concentration load solutions.
Higher mass loadings.
With intensified processing, a major goal is to identify technologies that offer high productivity, processing the same amount of mass through much smaller devices. For the most part, downstream processing of higher concentrations is advantageous, resulting in smaller intermediate product hold tanks, decreased loading
times onto chromatography resins, and potentially higher effective capacities during chromatographic operations – all key advantages of the approach. The biggest concerns would be potential competitive binding, which could reduce separation efficiency, and potentially introduce issues with protein stability. Chromatography resins and membranes for intensified processes should be capable of operating at high mass loadings while maintaining the expected separation resolution. For virus filtration, high mass loading of high concentration feeds could require more membrane area, unless the capacity of the virus filter can be increased. In addition, mimicking the at-scale process in a clearance evaluation would require a significant mass of product for small scale tests, and there is a higher likelihood that the virus spike itself might interact with the high concentration feed, which could, in turn, affect the filterability of the process solution.
Longer duration processing.
Intensified processing may involve targeting the same mass loading, but operating at lower flux for a longer duration. Depending on process duration, this is generally not expected to impact viral safety. Clearance evaluations would need to mimic this scenario, and include several starts/stops or process interruptions to mimic likely processing conditions. In addition, after several days of processing, bioburden could be a concern so manufacturers may need to think differently about bioburden control.
Connected/continuous processing.
Adoption of this strategy may have the biggest impact on viral clearance assessments as there will most likely be two unit operations running simultaneously both of which are designed to remove virus; for example, anion exchange chromatography and virus filtration. During a standard batch process, it is easy to isolate process steps and evaluate the viral clearance potential of each step independently. For a continuous process, it is more difficult to isolate each step, and instead of assessing clearance of a homogeneous batch, clearance would be evaluated across a step where the load solution might have a different composition at the start and the end of the ‘batch’. Additionally, evaluation of viral clearance will require specialized techniques and equipment.
Importantly, if an existing process for which viral clearance data have previously been generated is modified and intensified, that clearance data may no longer be valid. The increased concentration of the process intermediate, adjusted loadings on individual unit operations and slight modifications to the unit operation process window may impact the levels of viral clearance that can be achieved for individual steps. To assure the new process can deliver the expected level of viral safety, clearance studies should be performed using the new, more concentrated intermediate under the new process conditions.
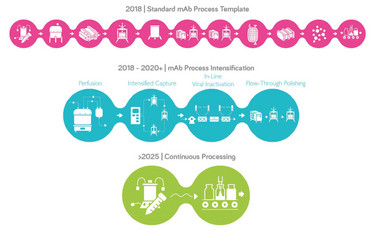
Viral safety strategies need to be re- examined as the mAb production template evolves to next generation bioprocessing.
Evolving the approach to viral safety
It is clear that next generation bioprocessing strategies impact the approach to viral safety. By connecting process steps, we can no longer evaluate the viral clearance of isolated steps, and process development should ideally include viral clearance evaluations. In addition, unit operations may be impacted by the previous step and the load solution to a step may not be homogeneous. This will require a creative approach to modeling newly developed processes to ensure they accurately represent the manufacturing operations. Furthermore, how we execute virus spiking studies may need to be reevaluated to minimize any negative impact of addition of virus to the test system.
At MilliporeSigma, we are focused on enabling advanced manufacturing through our BioContinuum™ Platform strategy that includes new process technologies and systems combined with new digital solutions. From a process technologies and systems perspective, we are developing solutions to support intensified fed-batch and perfusion processes, intensified capture, in-line viral inactivation, integrated flow through polishing, and continuous ultrafiltration/diafiltration. From a digital perspective, we are developing a new control platform and orchestration platforms that would be ‘future-ready’ to support additional digital technologies required to enable advanced manufacturing.
The way forward
From a viral safety perspective, monoclonal antibodies and recombinant proteins have a very safe track record. Although we may feel confident that next generation approaches are similarly safe, we need to demonstrate that intensified and continuous processes deliver the expected levels of viral safety. Doing this with confidence will require creativity in the development of novel spiking strategies and accurate small-scale models that reflect new processing conditions.
Undoubtedly, intensified processing requires the biopharmaceutical industry to think differently and more holistically. The ultimate benefits of the adoption of next generation approaches, however, far outweigh any challenges presented by technical, regulatory, and implementation aspects.
The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the US and Canada. MilliporeSigma, BioReliance and BioContinuum are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.
Kathy Remington is a Technical Consultant focusing on the BioReliance® portfolio at Merck. The life science business of Merck operates as MilliporeSigma in the US and Canada. Merck, BioReliance and BioContinuum are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.
Michael Phillips is Director, Next Generation Bioprocessing R&D, at Merck. The life science business of Merck operates as MilliporeSigma in the US and Canada.



















