Now You See It
What are the challenges and best practices in quality control for difficult-to-inspect products?
Andrea Sardella | | Longer Read

The manufacture of biologics is well established, but new challenges are emerging as protein products become more complex. In addition, the industry is now also pursuing personalized medicines, which present new challenges. Manufacturing hurdles are being widely discussed in industry, but a far less common – yet still important – topic is that of visual inspection. We have observed a sharp increase in the number of new therapeutics that are difficult to inspect, including suspensions, emulsions, lyophilized, and highly viscous products.
Each dose of a drug embarks on a long and complex journey between the point of manufacture and the point of administration. Every care is taken to ensure that integrity is not compromised, providing the necessary guarantee that the treatment patients receive is both safe and effective. A vital process during the journey is quality control, which encompasses the visual inspection of products. Irrespective of the type of primary container used or the nature of the drug held within, visual inspection provides assurances that the packaging and contents have been subject to rigorous review, facilitating detection of particles and cosmetic defects, and testing for leakage. This process is essential in identifying whether any potential issues during manufacturing or any adverse interactions between drug and container-closure system have adversely affected the purity of the contents. Though data on the risk of human exposure to infused particles is relatively limited, there is some evidence to demonstrate clinical implications for patients, from inflammation and infection to venous and arterial emboli (1).
Visual inspection can be characterized as a process with a simple and straightforward objective: safeguarding product quality. In typical applications, the process itself is relatively straightforward, particularly where the clarity of a drug product makes it an ideal candidate for inspection using inspection equipment, such as high-performance cameras, appropriate illumination levels, and container rotation mechanisms.
There is a growing emphasis, however, on products that are defined as difficult-to-inspect, which include concentrated suspensions and emulsions, lyophilized cakes and powders, and opaque and deeply coloured solutions. With biopharmaceuticals, the drive to address unmet needs in chronic diseases and rare cancers can often result in the formulation of complex and fragile proteins that require specialized manufacturing techniques. These proteins can be challenging from a visual inspection perspective for a variety of reasons. For example, with nanoparticle suspensions that present in opalescent and milky forms, the visual clarity required for imaging purposes becomes compromised; for high-viscosity products, inspection via pre-spinning becomes unsuitable because particle movement is severely restricted; and in the case of high-concentration vaccines, proteins can be prone to agglomeration, creating visual anomalies that can be falsely identified as external bodies.
The trend towards personalized medicine can also be a headache when it comes to visual inspection because of challenges relating to the highly tailored nature of the production process. Typically, batch sizes are small, levels of product variability are high, and products are often presented within soft bags, complicating handling and greatly limiting particle movements. And that means visual inspection equipment must be configured for each individual process, in contrast to commercial production environments designed for consistent, high-volume manufacture of transparent therapies packaged in clear primary containers.
Overcoming inspection challenges
For therapies that present as thick suspensions or emulsions, and where there is separation between liquid and sediments, the visual inspection process is even more difficult. Insulin is a common example of a therapy that presents in this way, requiring rehomogenization via spinning to ensure products undergo an accurate visual inspection. The precise level of inspection accuracy will, however, ultimately depend on the type of image processing technique employed in the camera-based inspection system – a point that is particularly relevant to high-speed inspection lines, where the rapid motion of cylindrical containers can be challenging for image capture and analysis.
Evidence has shown that line-by-line processing methods, based on continuous analysis, generate higher quality images with a finer level of detail when compared with frame-by-frame image processing. Even for very thick emulsions that are difficult to inspect manually, a visual inspection set-up involving line-scan cameras and appropriate lighting enables the detection of even white fibres, including hair fragments at 0.5mm and rubber fragments at 0.3mm. This is achieved because the line-scan camera avoids any time lapse between frames, and so can suppress the residual optical background “noise.” At a line rate of 30kHz, the defect detectability (DR) rate is 99.9 percent and the false reject rate (FRR) is less than 0.8 percent (2). In contrast, a matrix camera operating at 200 frames per second has been found to register a DR of 68.4 percent (2), while the FRR for frame-by-frame visual inspection techniques stands at around 10 percent. Of course, the lower the DR and the higher the FRR, the greater the risk of particles going undetected, and the greater the associated costs of business interruption.
The relationship between DR and FRR is particularly complex in the case of lyophilized products. Here, there are intrinsic physical variations in visual appearance that can occur between batches and within a batch – not all of which necessarily warrant rejection. These variations may be present in the form of differences in colour, structure (from dense to porous), topography (presence of skin, bumps, cracks and peaks), and the presentation of shrinkage, both uniform and non-uniform. The task for visual inspection is to maintain the highest level of DR accuracy while managing the higher risk of recording a false reject.
Let there be light
Evaluating container integrity for lyophilized products is also complicated, owing to the presence of dust, cracked cake, and product splash on the sidewall of the container. These elements can interfere with the analysis and increase the FRR.
Addressing this particular challenge requires the implementation of a specialized chip and crack (C&C) detection station, which uses the container glass as a light guide. Lights enters at a point far from the field of view, propagates within the container, and then exits with higher intensity at points of discontinuity, highlighting flaws while ignoring the presence of any benign elements. Particulate detection on the top and bottom of the cake also relies on the use of light and is achieved by exposing disparities between the optical characteristics of the various potential contaminants, the container, and the product itself. Glass fractions, for example, will not display the same diffusive properties as the cake and instead demonstrate a mirroring effect, which presents in an anisotropic angular dispersion. Notably, black particles display isotropic diffusion characteristics.
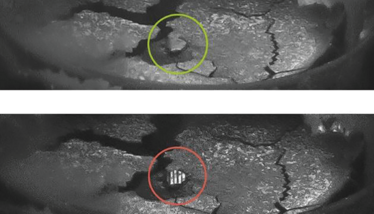
Figure 1: Lyo top inspection, glass fragments, and black particles
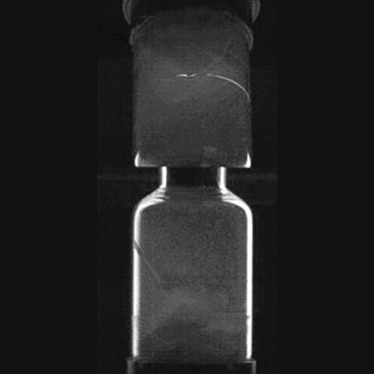
Figure 2: The glass wall of the vial acting as a light guide
The inspection of lyophilized products from the side angle presents less of a challenge but must accommodate the inspection of cylindrical surfaces. Using linear cameras, the full lateral surface of the cake can be imaged at very high resolution, providing a multi-dimensional view of the product and enabling defects to be identified even in awkward locations, such as under the stopper and crimping.
Just as lyophilized products require a specialized approach to visual inspection to sustain a high DR for contaminants, the same is true for highly viscous products. These therapies are increasing in popularity, driven by the benefits long acting injectables (LAIs) deliver in terms of patient convenience and compliance, and the reduced burden on healthcare professionals. Because of their higher levels of viscosity, however, these products are not necessarily suited to the typical automated inspection route based on spinning the liquid and measuring the subsequent particle movement when the container is stopped. At a dynamic viscosity above 4-5 centipoise (cP), this method of particle detection becomes ineffective.
Instead, high-viscosity liquids maintain constant rotation during inspection, with all potential contaminants tracked through 360 degrees. Where contaminants are located on the outside of the container, they will register at a higher speed, as well as a broader trajectory path and can, therefore, be disregarded. Analyzing the image against a dark background also accentuates the identification of contaminants such as white fibres, which can be distinguished from scratches through their speed of trajectory.

Figure 3: The apparent rotation speed of the potential contaminants is used to determine if they are inside or outside of the liquid
High viscosity as a by-product of protein concentration is one of the many attributes that make the growing field of biologics more challenging for the automated inspection process. These delicate products can be difficult to inspect as a result of attributes such as their high density and turbidity, while the fragile nature of their long-chain protein structure means they also have a higher sensitivity to shear force, UV light, and mechanical shock.
To cope with these challenges, new innovations in equipment are being seen. P-handling mechanisms, for example, smooth transitions up to 660 ppm, with a view to minimizing mechanical shock and limiting the cavitation effect induced in the liquid. Exposure to shear force is also reduced by accelerating liquids slowly and carrying out inspection while the container is in rotation at a steady speed.

Figure 4: Low acceleration pre-spinning

Figure 5: Detection while spinning dirty cancelled by correlation
Defining the future
Where next for visual inspection? The introduction of artificial intelligence brings great potential to visual inspection, raising the possibility of a system that can detect, measure, and quantify every particle within a container. AI-based machine-learning and deep-learning techniques are trained on data. Images of the particle in different positions can be automatically extracted, labeled accordingly, and fed into a central repository. Over time, this could become a self-managed intelligence centre that continually grows as it expands its “knowledge” of defects and its capability for recognizing defects.
The application of this deep learning approach has huge potential for the future of production. Here, flexibility and agility will be key attributes to accommodate the rapid and cost-effective production of smaller production batches in line with the trend for more personalized medicines. Bringing the learned intelligence of AI to modular robotic inspection stations could quickly deliver batch-level defect-detection capability, while introducing the potential for continuous improvement via sustained retraining and the ability to scale by increasing the number of units. Such a model would differ significantly from existing visual inspection systems, but would be driven by the consistent objective of maintaining the highest levels of productivity and product quality. Ultimately, patients must be the priority for innovation in drug therapies, and if those therapies present as difficult-to-inspect, then organizations have a duty of care to respond in an equally imaginative, innovative, and personalized way.
- SE Langille, “Particulate matter in injectable drug products: evaluation of risks to patients,” Pharmaceutical Technology in Hospital Pharmacy, 1 (2016). DOI: 10.1515/pthp-2016-0004
- B Lopez Romero, “Assessment a, selection and implementation of a replacement syringe inspection machine” presented at the MSD Technical Symposium; October 10, 2016
Pharma Inspection Product Development Manager at Stevanato Group



















