Optimizing AAV Manufacture
With the potential to cure genetic disorders rather than alleviate symptoms, gene therapies look set to revolutionize the field of medicine. Adeno-associated viruses have emerged as the vector of choice for delivering therapeutic genes to target cells, but manufacturing processes need to be improved and optimized to unlock their full potential.
Orjana Terova, Zoltan Gulyas |
sponsored by Thermo Fisher Scientific
Over the last decade, significant progress has been made in our ability to deliver therapeutic genes to target cells. Medicines that are able to replace faulty and missing genes are genuinely life-changing.
Despite the relative immaturity of the field, two gene therapies have already been approved by the FDA (Luxturna® and Zolgensma®), and the pipeline looks strong; the FDA expects that approvals for cell and gene therapy products will rise to 10–20 per year by 2025. Right now, gene therapies are targeting orphan diseases, especially in children, but they have the potential to treat central nervous system related disorders, such as Alzheimer’s, Parkinson’s or Huntington’s disease.
Expectations are high, but there are many challenges on the road ahead; as gene therapies are looking to treat larger patient populations, there is a concomitant need to increase the manufacturing scale, and improve productivity and process control.
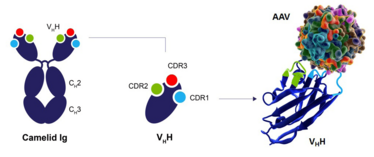
CaptureSelect technology is based on the single N-terminal domain of Camelid IgG, the VHH fragment. Camelid-derived immunoglobulins are naturally devoid of light chains. The small size of the VHH fragments allows for binding to epitopes of the target molecule which are difficult to access by larger immunoglobulins. Overall, the VHH fragments offer high specificity, affinity and stability.
The vector of choice
Selected viruses have been successfully engineered into smart vehicles to deliver DNA to target patient cells. These viral vectors lack any viral genes but contain DNA sequences of interest for various therapeutic applications. In particular, recombinant adeno-associated viruses (AAVs) have emerged as the vector of choice for many therapies for several reasons.
First and foremost, AAVs are generally considered safe, as they are nonpathogenic and non-toxic, and have inherently low immunogenicity, when compared with other viruses. Scientists have identified 13 naturally occurring serotypes so far, and each of them has different tropism (i.e., ability to target specific cell types), which enables selective transduction of specific tissues and organs. Companies are actively developing novel, engineered capsids to further improve tropism, and thereby increase the potency.
From a more practical standpoint, AAVs are relatively simple to manufacture, and these vectors have no lipid envelope as found on retroviruses and lentiviruses, so they are more stable, and able to withstand the typical process conditions used for protein purification such as low pH and high salt.
The potential of AAVs to treat wider patient populations and target more common disorders is somewhat limited due to the manufacturing and scale-up related challenges. The majority of gene therapies in late clinical phases are the result of first-generation processes that started as research projects in academia or hospitals years ago. In these settings, vector production is often performed by the classical tools and methods such as adherent cell cultures on plates or cell factories, sonication for cell disruption and ultracentrifugation for purification. Most of these techniques are either not scalable or can only be scaled out.
Essential evolution
Today’s gene therapy developers are using scalable techniques from the beginning of development, and recognize the need to not only improve productivity, but also process robustness and reproducibility. At the same time, regulatory agencies are expecting increasingly established product control and product characterization.
The monoclonal antibody (mAb) field was in a similar position not so long ago. It, too, had to evolve and mature, and can serve as a “role model” to the gene therapy field. Today, suspension cultures are used with high cell density to achieve high titers, and purification is performed by multiple chromatography steps including the highly selective affinity capture and the orthogonal polishing steps. All tools and methods are GMP-compliant, and the product is extensively characterized to ensure its safety and efficacy.
Broadly speaking, the gene therapy field needs to follow a similar path, which requires significant investment of time and resources, while meeting speed-to-market needs. Though the path is similar, we must recognize that we cannot simply “copy and paste” solutions from the biopharma industry as these were designed with a different mind-set for different molecules. When it comes to optimizing AAV processes, there is much work to do.
In terms of upstream processing, gene therapy developers are still seeking reproducibility and looking to push productivity orders of magnitude higher than the current standards. Improved packaging mechanisms are also needed to boost the percentage of full capsids (those that contain genetic material) versus empty capsids (which have no therapeutic value).
Turning our attention downstream, developers need scalable purification methods with high selectivity towards the molecule of interest, and high recovery to make sure not to lose the produced material.
Surrounding these elements is the need for accurate and reliable analytics that enable DoE-based process development, product characterization and quality control.
Recognizing the need for progress across the board, the Thermo Fisher Scientific bioproduction team is active in all these areas (see: “Innovation for All”).
Innovation for All
In Thermo Fisher Scientific’s BioProduction Division, all business units are devoted to bring solutions to the gene therapy field. The cell culture team is focused on the development of suspension cell lines, media and additives to ensure high productivity upstream. The single-use team develops bioreactors for suspension culture (both in batch and continuous mode), and highly customized single-use bags that are gamma irradiated for immediate use in clean rooms for closed processing. The purification team is focused on highly specific affinity resins to establish platform capture for AAVs. We also offer ion exchange resins to enable full capsid enrichment and additional impurity clearance. Finally, the pharma analytics team have developed highly sensitive assays for process-related impurity and advantageous agent detection. We have just launched a residual DNA detection kit for HEK-293 cells, and an Sf9 specific kit is in the works. Lastly, our dedicated viral vector services team has extensive expertise in clinical and commercial manufacturing of AAV, to progress programs from early to late phase development and commercialization.
AAV Affinity Chromatography – the game changer
Focusing on purification, many companies have moved away from ultracentrifugation over the past few years and established multiple chromatography steps including ion-exchangers and hydrophobic interaction resins to achieve the required purity. In addition to the lengthy processing time and raw material cost, however, such multi-step processes generate cumulative yield losses. Moreover, process development lead times increase, hindering speed-to-market.
Affinity chromatography can overcome most of these challenges, as it can selectively capture the product of interest from crude material, providing high purity and yield in a single step, and robust methodology with less need for process optimization. This highly specific separation delivers significant improvements to downstream processing by reducing the number of purification steps and maximizing productivity.
Affinity chromatography is already a key element of the purification platform for monoclonal antibodies (consider Protein A), and specifically from Thermo Fisher Scientific’s standpoint, our CaptureSelect™ team has been developing affinity solutions for over 15 years, enabling a similar paradigm shift in the purification of antibody derivatives, recombinant proteins and now viral vectors. Due to the larger size of viral vectors, the affinity ligands are immobilized on Thermo Scientific™ POROS™ base beads, which are extremely suited for the purification of larger molecules (see: CaptureSelect and POROS Up Close).
CaptureSelect and POROS Up Close
CaptureSelect technology is based on a strong foundation of over 15 years of experience in developing affinity ligands and producing resins for GMP manufacturing. The platform uses the variable domain of the heavy-chain only camelid antibodies called VHH – a single domain with a size of 15 kDa that provides full functionality in antigen specific recognition and high affinity binding. Their compact structure and the lack of light chains also results in increased stability, which allows them to withstand a wide variety of process conditions when applied as affinity ligands.
For large target molecules such as AAV, the CaptureSelect ligands are immobilized on the POROS backbone, which is a rigid, polystyrene-divinylbenzene based solid support with large pore structure to ensure high binding capacity and a more efficient purification process. The large pore structure of the POROS resins results in reduced mass transfer resistance and as linear velocity increases, capacity and resolution decline very little. This leads to improved process productivity.
We currently offer three POROS CaptureSelect AAV affinity resins – AAV8, AAV9 and AAVX. As their name suggest the POROS CaptureSelect AAV8 and AAV9 resins were developed for the indicated serotypes, while the POROS CaptureSelect AAVX resin works for all naturally occurring serotypes as well as engineered capsids. This allows our customers to use it as a platform capture step in all their AAV projects (similarly to Protein A for mAbs). Based on the feedback we received since it launched, the AAVX resin is largely fulfilling the industry’s expectations, enabling high purity in a single step, offering process consistency from lab to production scale. Lastly, the performance of POROS CaptureSelect AAV affinity resins is maintained even at high flow rates, thereby enabling increased productivity and process flexibility.
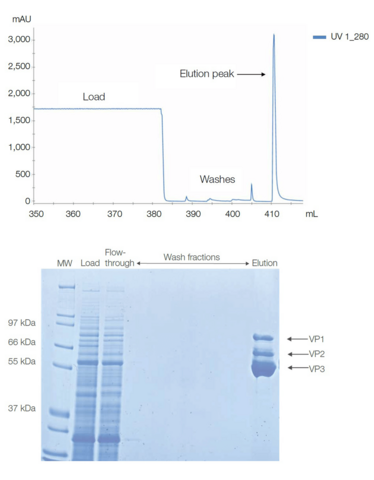
Chromatogram showing elution peak of rAAV6 purified on POROS CaptureSelect AAVX affinity resin (top).
Fractions from AAV6 purification run on a Coomassie stained gel. The capsid proteins VP1, VP2, and VP3 are indicated (bottom).
Team players
Even when using affinity chromatography, users still need to perform process optimization to ensure high purity and recovery. This work is more crucial in the gene therapy processes, where the current product and process understanding is limited, and the “plug and play” approach often leads to lackluster process performance. The importance of optimization goes beyond the affinity capture step (see: “Realities in the Field”).
Realities in the Field
Our dedicated team of field applications specialists are more than happy to answer questions and help solve problems. Here are some advices and points to consider regarding affinity capture of AAVs.
- Process steps between harvest and capture chromatography are often neglected or not properly optimized, but the feed-stream quality can have a profound impact on purity, yield and process performance. Removal of all insoluble components by depth and membrane filters is important to avoid backpressure issues and column clogging. We also recommend soluble impurity reduction by various techniques (such as endonuclease treatment, flocculation, tangential flow filtration, and/or various chemistries on solid support) as much as possible prior to affinity capture.
- Low product concentration in the load can cause earlier break-through and thereby resin capacity loss. Feed-streams can be concentrated by TFF, which also provides impurity clearance and reduces the loading time.
- If capsids are present in the flow-through, increasing the residence time may be able to mitigate this.
- Root causes for low recovery of the capture step could be caused by under-loading the column due to insufficient product quantities (loading around 1E12 vg/mL resin or below, which is 2-3 logs lower than the AAVX capacity), lack of elution efficiency, and/or overestimation of load concentration. Column volume reduction, eluting in upflow and optimizing the elution (evaluating different buffers, pH and additives) can mitigate the product loss.
- Insufficient eluate purity can be resolved by incorporating and optimizing intermediate washes between load and elution.
- If the affinity resin is meant to be reused, cleaning optimization should be performed to avoid carry-over issues. We recommend an acidic strip followed by cleaning with a chaotropic agent, such as guanidine hydrochloride. Concentrations and contact times are process dependent, but upflow direction is always recommended. Please note that our AAV affinity resins are compatible with up to 25mM NaOH only.
In this rapidly evolving and challenging field – and with such high expectations – teamwork is more important than ever. Upstream, downstream and analytical experts need to be in constant communication, and combine efforts to move the needle. This is why our team of field application specialists is keen to engage and collaborate with gene therapy developers on technical matters – to discuss recommended conditions, troubleshoot problems, and brainstorm on challenges. We are eager to learn, and as our knowledge base grows, we become better equipped to provide more efficient support and develop next-generation solutions that can help companies overcome the productivity and scalability challenges.
At Thermo Fisher Scientific, we are committed to provide the tools and services needed to manufacture AAV drug products, smoothing the path to commercialization and helping to bring life-changing gene therapies to the clinic faster.
Orjana Terova is Senior Product Manager, Purification, and Zoltan Gulyas is Senior Field Applications Specialist, Purification, both at Thermo Fisher Scientific.



















