Protein A: a Question of Affinity
Is there a future for Protein A in affinity chromatography? Some believe not, but others point to the long-established benefits of Protein A resins and the developments and advances that continue to be made in the field.
sponsored by GE Healthcare

Scientists and process engineers have been discussing potential alternatives to Protein A for some time – and debating whether or not antibody manufacturers will still be using Protein A at all in the next 20 years. Some see the costs of affinity chromatography and Protein A as a hindrance, but there is little question that it gets the job done. Given that Protein A is the result of millions of years of evolution, is it even possible to synthesize something better?
Affinity chromatography is used in the vast majority of antibody processes, and is used increasingly in processes for antibody fragments. It is generally the first step of the purification train where the goal is to remove the majority of contaminants from harvested cell culture fluid and to concentrate the product before any subsequent purification and polishing steps.
Here, Jonathan Royce, BioProcess Senior Product Manager of Antibody Affinity Resins at GE Healthcare, considers how trends and challenges in affinity chromatography are driving innovation in the field.
Some believe that Protein A will be replaced in the future – do you agree?
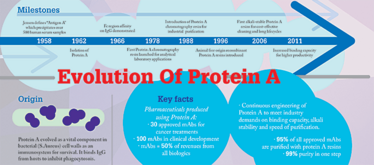
I have heard many people talking about the quest for the “holy grail” that will replace Protein A, but I believe that Protein A is the grail! We’ve been using Protein A for decades and it has a selectivity that is hard to top thanks to natural selection. Protein A is a naturally occurring protein that exists on a specific strain of bacteria – the bacteria has developed its own defense system against an antibody immune response over millions of years of evolution. In addition, over the last 50 years, scientists have used engineering skills to provide other benefits to Protein A based affinity chromatography. Synthesizing something that can compare with that kind of development is always going to be difficult, especially when there is still interest and effort in maintaining developmental momentum. I believe that Protein A will continue to have a dominant role for a very long time.
In general, Protein A resins have not become more expensive from generation to generation, and are ultimately providing better process economies to end users as time goes on. For example, a good article was recently published by scientists from Amgen (1) examining the entire marketplace for Protein A resin. The article showed that, if you look at the development of Protein A resins over time, there is a steady improvement in their productivity, which ultimately leads to improvements in the cost structure for the end user.
Importantly, Protein A is not the only aspect of affinity chromatography that continues to improve – we are also seeing developments in hardware in terms of automation and sanitary design, as well as efforts to demystify the task of packing columns reliably.
What are the alternatives to affinity chromatography and how do they compare?
There are many alternatives to affinity chromatography out there; just as an example, some companies use two-phase separation, which creates a phase-separation barrier between an aqueous phase and a non-aqueous phase, and then concentrates the antibody into one of those two phases. It is also possible to perform precipitation of antibodies and there are certainly people who have developed processes that use traditional chromatography without an affinity step.
All of these have been tested and they are all functional from a technical standpoint, but I don’t believe they provide the same levels of simplicity, yield and purity as affinity chromatography. The one downside of affinity chromatography and Protein A is that it can appear to be expensive compared with other chromatographic steps. The ligands themselves are recombinant proteins and the process to produce them is complex. However, if you sit down and calculate the costs of the technology and what you gain in terms of savings in process development, high yields and reduced time to market, I think most people will agree that affinity chromatography is actually cost effective.
What important trends have you noted in the affinity field?
The general trend over the last two decades has been a steady push to increase the capacity of affinity resins. Traditionally, it is fair to say that affinity resin has lagged behind ion exchange and other types of resins that rely more on chemical interactions in terms of capacity (or how much mass of antibody or antibody fragments can bind per liter of resin used). This reduced capacity stems from two factors that limit the diffusion of antibodies into the resins: i) the ligand used is quite large compared with ion exchange, and ii) the binding of affinity ligands to target molecules is relatively strong. Nevertheless, significant progress has been made in boosting the capacity of affinity resins, so now scientists are turning their attention to the process itself: how can we move from a traditional batch process to a continuous or semi-continuous capture step? Rather than processing one batch at a time with a relatively large installation of equipment and resin, it’s possible to shrink the equipment and columns, and to use more frequently: continuous processing. This is one area that we are examining in great detail at GE Healthcare – in terms of both equipment and resin.
Another growing trend is the search for affinity resins that are more alkali-stable. Why? Because we want to clean chromatography resins with relatively high concentrations of sodium hydroxide to remove proteins left on the resin and control bioburden. Today, we have affinity ligands that are stable at concentrations of 0.1–0.5 M, but there is a desire to push stability further; ion exchange resins are usually cleaned using 0.5–1 M sodium hydroxide. If affinity resins were developed that could withstand the harsh chemicals being used on subsequent steps, it would simplify how people manage clean-in-place (CIP) solutions. Perhaps another important advance in the industry is the development of pre-packed chromatography, which seeing growing interest in the industry. Users rely on vendors to provide ready-to-use columns that can be discarded at the end of their useable life.
Looking at the world of antibodies in general, I would say that the industry is seeing diversification of the antibody pipeline. Fragments have become more popular and represent a larger proportion of earlier clinical phases than they did 10 years ago. Fragments, by their very nature, lack the Fc region of the antibody to which Protein A binds, leading to the development of other affinity solutions; for example, Protein L and Protein G based resins, which bind other moieties of the antibody structure.
How are the growing number of antibody drug conjugates and bispecific antibodies affecting purification processes?
The purification of antibody for an ADC isn’t very different to that for a typical antibody – and a lot of the ADCs in development or that have been launched are based on antibodies that were developed 10 or 20 years ago. However, there are certainly challenges after conjugation, the most common being how to separate the ADC from free antibodies or cytotoxin. The separation is made more challenging because you are working with cytotoxic agents under special conditions. There is a lot of focus on how to perform these separations in a closed manner, with minimal handling of the raw materials, which has led to a strong preference for single-use technologies.
In some cases people have solved some of these separation challenges by using filtration steps, but they can also be solved by using scavenging resins that are built into pre-packed chromatography columns or by using membrane absorber technology. There is no standard solution yet, which means that each project requires customization, but in time I think we will see a greater level of standardization as the industry begins to understand what works reliably for these complex products.
Bispecific antibodies have a different set of challenges because they are very much like fragments and their structures can be diverse. There are more than 40 different bispecific antibody structures under development today. Some can be addressed with Protein A, some with other kinds of ligands, while others require individual purification processes based on orthogonal techniques, where process development must be performed from scratch each time.
There is no question in my mind that bispecifics will create a larger market for customized purification solutions, where vendors develop a specific resin for a specific molecule, such as customized versions of Protein A resins or even customized chromatography techniques. As an example, GE has previously developed a specific variant of one of our Protein A resins for a customer who had a unique, patented bispecific antibody structure. With an increasing number of these kinds of products coming through company pipelines, it will be crucial for resin manufacturers and antibody manufacturers to work together.
What are your tips for choosing the best purification equipment and processes?
It is important to try to think ahead – to anticipate how manufacturing will look in the future. What is your company’s plan for manufacturing? How do you want your process to look? You need to understand what equipment you already have available, what needs to be acquired to implement the process, and what the ultimate goal of the manufacturing process is. Is it going to run at maximum productivity? Do you need to minimize the investment in terms of capital equipment? Do you want to minimize the amount of time spent on process development from project to project? If you spend quality time thinking about these questions then your answers will establish much of the criteria for choosing the right solution.
One thing that is easy to underestimate, if you are not already working this way, is the value of a platform process. Nearly all of the major antibody manufacturers have settled on a platform process that they leverage from project to project. And there is a very good reason for this: when you have a platform, you do not have to constantly repeat process development each time a molecule comes into your portfolio. A platform will probably never be 100 percent optimized, but it will be good enough to satisfy the needs of most projects – and the benefits of having a standardized set of operations will generally outweigh the incremental gains you could obtain by optimizing every individual process.
- GR Broton and KK Mehta, “The role of more than 40 years of improvement in protein A chromatography in the growth of the therapeutic antibody industry”, Biotechnol. Prog., 32, 1193-1202 (2016).



















