Scaling Gene Therapy Challenges – Together
Understanding AAV production and characterization processes – and establishing strategies for success
Catherine Buchere, Kathrin Teschner | | 7 min read
sponsored by Sartorius
Viral vector gene therapies show great promise for treating a myriad of diseases, and the excitement across the field is palpable. As more therapies approach commercialization – and as interest grows in using gene therapies for broader indications – biomanufacturers will need to establish manufacturing strategies that help them to respond to the anticipated surge in demand. Gone are the days of targeting only rare diseases with resulting low demand – the gene therapies of the future will demand optimization of all aspects of manufacture and characterization.
As you will know, the leading viral vector for gene therapy is the adeno-associated virus (AAV) – a small non enveloped, single- stranded DNA virus with a diameter of 18–25 nm. Thanks to their low immunogenic profile, which complements low pathogenicity during gene delivery and reinforces safety, AAVs are a powerful delivery vehicle. Moreover, different AAV serotypes can exhibit specific tropisms for specific organs and tissues of the body to improve targeting (1) – and new serotypes are continuously being discovered.
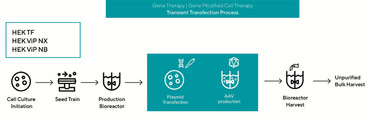
Figure 1. Overview of Transient Transfection Using AAV Vectors
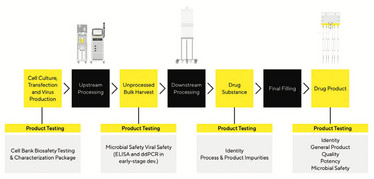
Figure 2. Biosafety and Characterization Testing Methods Are Required at Every Stage of AAV Product Manufacturing
Understanding the production challenge
The production of recombinant AAV vectors with high purity and potency – crucially, while maintaining good yield – is complex. Indeed, when it comes to manufacture of a given viral vector, there is no standard and robust approach to upstream and downstream processes.
Most commonly, AAV production employs a three-plasmid transfection model to encode the gene of interest, packaging rep/cap, and helper genes. This transient triple transfection allows for yields in the range of ~103–105 vector genomes per cell – and the process is well established, being widely adopted in research laboratories. But adapting the process to a bioreactor environment for scale up can present challenges, especially when adherent cell cultures are still used for production.
These scale up challenges have led many process development teams to focus their manufacturing strategy on developing a producer and packaging cell lines, with the components necessary for rAAV production integrated into the genome (either partially or totally). This approach brings scalability benefits and avoids the critical step of transfection, but there are drawbacks – not least the time and upfront process development required to develop such a stable cell line – as well as the financial investment. Importantly, each serotype/vector combination requires the generation of a unique cell line.
It’s clear that both approaches have disadvantages as well as advantages – but, right now, they remain the main options for viral vector production. The choice between them typically depends on the timeline, budget, and the gene therapy being developed.
Optimizing Through Serum-free Media
Scale up can be challenging and bottlenecks are amplified when using adherent cell lines. One key to the success of the AAV production process upstream is selection of the optimal media for the cell line expression system. To sustain growth and productivity, high-performing media should mimic the production cell’s natural environment, such as energy source (glucose), vitamins, amino acids, trace elements, lipids, hormones, and salts.
Currently, two main cell lines are used to produce AAV gene therapies: human embryonic Kidney 293 (HEK 293) and insect cells isolated from the fall armyworm Spodoptera frugiperda (Sf9). One of the approaches to circumvent processing bottlenecks and scalability challenges is to adapt cell lines to suspension cultivation, and then transition to a bioreactor on a large scale (50 L) at the start. Suspension cell processes in serum-free media can be operated in batch or fed-batch mode to enable high yield.
Advantages of using serum-free media include more consistent performance, increased growth and/or productivity, better control over physiological responsiveness, and reduced risk of contamination by serum-borne adventitious agents in cell culture.
Sartorius is a well-known supplier in the biotech world and offers technologies for every phase of the biopharmaceutical value-added chain. Known as a pioneer in single-use bags and filters, Sartorius has massively invested in the science and manufacturing of the cell culture media for gene therapies, protein-based, and advanced cell therapy modalities.
For gene therapy applications, we offer free samples of our HEK media kit. Request yours here with Sartorius
Know your analytical methods!
Experts at Sartorius have produced a poster titled, “Analyzing AAV – A Story of Problems and Solutions” (2). Although AAV has many advantages for gene therapy and has thus gained an outstanding reputation, the efficacy and safety of AAV-based gene therapy is dependent on an optimal manufacturing process followed by robust characterization processes that reveal titers of capsid as well as the vector genome.
Various methods are available for vector characterization – but again, we face a conundrum of advantages and disadvantages, which are shown and compared in our poster. ELISA is probably the most used method to determine the total number of capsids in a sample, but the value is often accompanied by a high coefficient of variation (CV). An additional method is needed to determine the proportion of full capsids, and the genomic titer is usually determined by qPCR (although we prefer newer methods, such as ddPCR due to greater robustness). Genomic and capsid titers divided subsequently give the full:empty ratio – but this result is, of course, affected by the combined error of the two methods.
Another analytical technique that can be used for characterization is size-exclusion chromatography with multi-angle light scattering detection (SEC-MALS). This approach allows determination of several AAV quality attributes in a single measurement, including total capsids, full capsids, and aggregation. The disadvantages? In addition to the lack of high throughput, the sample must be purified beforehand, which makes in-process control more difficult. Additionally, user-related deviations must be considered – as must the measurement error of the method.
Is there a third option to consider? Determination of the genome and capsid titer using parallel purification of the sample is also possible using an affinity chromatography method we developed in-house (outlined in our poster). It has high precision and compares well with the results of the other methods. But it too has a drawback: When titers are low, the sample volume needed for reliable analysis is high...
It’s also worth remembering that, depending on the development phase of the gene therapy, analytical priorities may vary. For example, during process optimization, the need for in-process control and high throughput is high. But to ensure important quality attributes in the in vivo gene therapy, other factors, such as precision and robustness, become the highest priorities and thus decisively determine which methodology should be chosen.
So, what is the overall message of our poster? Know your methods! All methods for determining AAV quality attributes have advantages and disadvantages. Without an existing certified reference, each method can be optimized in terms of precision – but which method ultimately represents the true titer? Right now, the industry doesn’t have all the answers. But by combining different measurement methods perhaps we have a chance of getting as close as possible to the true value from a large data set.
Scaling the mountain together
The process of understanding viral vector production is a mountain, where complete knowledge represents the summit. Despite years of research in the field – and especially the focus on AAV in recent years – the industry is not yet at the summit, but we are inching closer. In fact, the scientific community has been climbing for years and we have made it through some particularly difficult terrain, but we must keep climbing.
Notably, the biggest mountains are not conquered alone – it almost always takes a team. To understand viral vector production – and more specifically AAV production – we need to make a concerted effort to combine methods and deploy exper tise from different fields. And that’s exactly the approach we have tried to take at Sartorius. With our poster, we wanted to show how customers can combine different methods to gain even more knowledge – and to keep everyone moving in the right direction. One day, we will reach that summit – together.
- S S Issa et al., “Various AAV Serotypes and Their Applications in Gene Therapy: An Overview,” Cells, 12 (2023). DOI: 10.3390/cells12050785
- Sartorius, “Analyzing AAV – A Story of Problems and Solutions” (2022). Available at https://bit.ly/3o6axhU
Product Manager Virus Based Therapeutics
Manager of Viral Vector Technologies, both at Sartorius



















