
Search and Destroy
Serialization will certainly help in the battle against counterfeiters, but there are other steps that manufacturers can take.
According to the International Anti-Counterfeiting Coalition, the illicit market of counterfeit drugs has a global value of more than one trillion Euros – and consultancies in Europe estimate that this may grow to more than two trillion Euros by 2020 (1). Globalization has created the ideal conditions for the counterfeit drug industry to boom, with sales made easier through lower transportation costs and little or no marketing costs for counterfeiters in comparison to legitimate pharmaceutical businesses. Countries that lack effective drug regulatory agencies are seen as particularly easy targets by counterfeiters, but counterfeit medicines have also slipped into legitimate supply chains in Europe and the US too. Some pharma companies have retaliated with well-thought out strategies that involve strong investments in anti-counterfeiting technologies (both overt and covert) and awareness campaigns that educate consumers on how to spot counterfeit medicines – and how to report them – as well as how to buy medicines safely online. Many big pharma companies have dedicated anti-counterfeiting teams, but small and medium enterprises are often much less likely to respond to the dangers because of a lack of resources.
Action against counterfeits from regulators and authorities, although potentially highly effective in some ways, has also served to constrain genuine supply chains through measures that are not unified and that lead to the need for new, complex (and expensive) systems. Both the EU and the US have implemented legislation around serialization, but the two systems are quite different. The US’ Drug Supply Chain Security Act (DSCSA) is being rolled out in different phases (the final phase is slated for 2023), whereas the final deadline for the EU’s Falsified Medicines Directive (FMD) is February 2019. Both systems require slightly different information and give no guidance on what serial number systems or data collection systems should be used. Companies have been forced to expend significant resources on dissecting the new regulations and implementing appropriate solutions – and there are huge concerns about how ready the industry is for the impending deadlines. Industry surveys show that only 51 percent of pharmaceutical manufacturers are expected to be ready to provide serialized products by the deadlines outlined within serialization regulations (2); in the EU, many member states won’t even have a national system in place by the February 2019 deadline for providing the information. Under the FMD, an “EU hub” will collate the serial data of drug products from across the EU, but each country will also require a national hub that will allow local pharmacies in each country to verify a dispensed product against national records. To date, only nine EU countries have their National Medicines Verification Organization (NMVO) ready (2).
Dealing with data
Despite the challenges, every company – whether large or small – has a duty to help deter counterfeiters. On a company level, the ability of pharma manufacturers and repackaging organizations to provide large volumes of serialization data within different countries is highly variable. One of the main hurdles for companies is simply identifying the right technological serialization systems. Drug manufacturers have been working with machine and software vendors for years to conduct research and evaluate the most efficient solutions, such as whether to opt for integrated solutions from one supplier, or systems that can coordinate, as painlessly as possible, existing production and logistical processes. When it comes to serialization and information management, there are five levels:
- Level 5: the highest possible level used for governmental reporting systems
- Level 4: corporate IT solutions that manage serialization and business processes – often referred to as the enterprise level.
- Level 3: localized systems within the manufacturing plant or distribution center
- Levels 1 and 2: inputs and line controllers into the level 3 servers and file systems, including vision controllers and packaging machinery.
For pharma companies, Level 4 is often considered the most vital element of the data lifecycle, as it includes the collection, management and verification of serialization data. It is the level where the big data lifecycle starts the journey to/from authorities and to/from wholesalers/manufacturers, and the system must manage manufacturing, packaging, warehousing and distribution. Large Level 4 software developers tend not to be amenable to sharing their proprietary software, so manufacturers must choose between two solutions: either serialize on their own with a custom solution, or use a cloud-based solution. Companies tend to be split between using cloud-based or hardware storage. Neither choice is necessarily “better” than the other, but it is important to judge which works best for your individual circumstances.
Despite the apparent conundrum of choosing the right system, it’s worth noting that the main software products available on the market for serialization, in general, do not vary greatly in function.
In the early days of serialization, initial discussions focused on the need for “aggregation,” where a shipper needed to provide 100 percent accurate linking of the serial codes within each case to the serial code of the case itself. This seems to have been refined based on practicality and, from an economic standpoint, most companies have agreed that aggregation makes sense to be placed on the packaging line. Large shipping units, such as pallets or big boxes and bags are far easier to scan as a single unit and for their codes be introduced to enterprise resource planning (ERP) systems. Without aggregation, it would be necessary to scan every item individually on a pallet, which could be more than 10,000 units.
More recently, industry attention is moving to what takes place at third-party logistics providers (3PLs), as these are generally seen to be potentially exploitable points in the supply chain because of their lower levels of GMP. Some manufacturers have also realized that the optimal point for aggregation may be in the warehouse, as orders are put together for shipment there, rather than at the end of a packaging line. The main challenge of moving aggregation to this step is that manufacturers usually have no distribution agreements with secondary wholesalers. In addition, some wholesalers may work with non-pharmaceutical products, and their staff may not have any specialized knowledge or experience in pharmaceutical warehousing or the quality assurance management of serialized products. The consequences of not having dedicated staff at this stage of the supply chain can be disastrous for companies that do not apply aggregation (aggregated batch sizes require less man hours). I believe that both the EMA and FDA have recognized the importance of manual labor and of shop floor activities and will be looking towards building up strong quality assurance systems within warehouses and supply chain departments.
Beyond barcodes
Serialization is not enough to crack the counterfeit nut, but there are also a wide variety of other solutions that can help deter counterfeits, such as specialized cartons, labels, overwrapping, tamper-evident tape, holograms, and color-shifting images – and even on-dose identifiers, which mark individual tablets. Tamper-evident packaging has been flagged by many companies as a dependable method of slowing the movements of the counterfeit industry, enabling fake products to be intercepted and verifying the authenticity of products to end-users (4).
Today, many consumers will knowingly buy counterfeit products, such as fashion accessories and clothes, but counterfeit medicines pose serious health risks. The true extent of the problem is unknown, and despite efforts to make counterfeiting more difficult, those involved in the practice have evolved their methodologies. Serialization and track-and-trace technologies promise to help manufacturers fight counterfeits by providing more traceability than ever before over medicines in the supply chain. But we can’t stop there. Companies must not be complacent and must also continue to invest in new solutions. The way to beat the counterfeiters ultimately lies in making tampering and counterfeiting more challenging and less profitable.
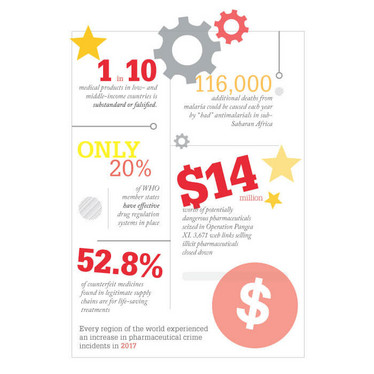
The Numbers Speak for Themselves
Counterfeit and falsified drugs are one of the most pressing global public health crises of the twenty first century. Counterfeit drugs may be contaminated, be in the wrong dose, or contain the wrong or no active ingredient – counterfeit drugs have been found to contain rat poison, brick dust and arsenic. The rigor of legal control within a country often affects how easily counterfeit medicines can be distributed, which is why they are most prevalent in certain geographic areas.
Sources for infographic: WHO, IFPMA, Pharmaceutical Security Institute, Interpol
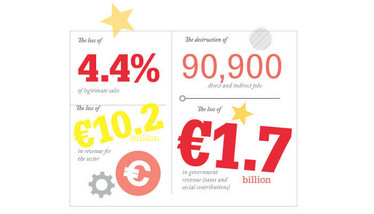
Fakes in Europe
In the US and Europe, less than one percent of counterfeit products are sold as medications. But the costs still hurt. A report released by the European Union Intellectual Property Office in 2016 revealed that fake medicines in the EU caused:
- World Health Organization Bulletin, “Growing Threat of Counterfeit Medicines,” (2010). Available at bit.ly/2PGfIPk. Last accessed January 15, 2019. EMVO, “Announcement – One Additional Country Connected to EU Hub” (2018). Available at bit.ly/2VlndyJ. Accessed December 18, 2018.
- Official Journal of the European Union, “Commission Delegated Regulation (EU) 2016/161” (2015). Available at bit.ly/2kr127a. Accessed October 30, 2018.
- FDA, “CFR - Code of Federal Regulations Title 21” (2018). Available at bit.ly/2EUhQ5f. Accessed on October 30, 2018



















