Small but Mighty
With numerous applications for small molecules – from improving solubility and bioavailability to making formulations suitable for inhalation – micronization is a versatile technique. But how does it work and what challenges does it pose?
Salvatore Mercuri | | Technology

Micronization reduces the particle size of APIs down to a few micrometers to enhance bioavailability. The technique is often performed using a jet mill; a compressed gas source expands at the outlet of a series of grinding nozzles, accelerating API particles in a spiral vortex flow path within the grinding chamber of the jet mill. Then, comminution, caused by particle-on-particle collisions, starts taking place for the particles in the flow field. And each particle is subject to centrifugal and drag forces: large particles are dragged to the outside of the milling chamber via centrifugal force while small particles migrate to the center mill outlet. From there, the smaller particles, dispersed into the gas stream, are discharged into a cyclone filter and progressively collected for use.
The jet milling process is controlled by three operating parameters: material feeding rate, feeding pressure, and grinding pressures. At a constant feeding rate, the increase in the grinding pressure leads to increased particle size reduction. The process is also thermally stable – with the heat produced by a jet mill dispersed by adiabatic cooling as the gas expands – compared with a mechanical mill that generates heat without dispersing it.
Micronization can be used for particles that need to be reduced in size to improve solubility, dissolution rate, or processing. It is also required for dry powder inhaler (DPI) applications, which have specific size requirements for lung and central airway delivery. Recently, these formulations have seen significant growth due to the increasing number of patients diagnosed with respiratory illnesses, such as asthma and COPD. Additionally, the lung’s absorptive capacity continues to be explored as an attractive delivery point for both local and systemic applications. The ideal spherical equivalent diameter for an inhaled API is usually a Dv50 of 3 µm (meaning 50 percent of the product is composed of particles under 3 µm). Particles designed to target areas deeper into the lung have to be even smaller.
Beyond inhaled drug products, micronization can also be used for many other types of treatments including oral medications, topical creams, suspensions, and eye drops.
Although any solid material – even diamonds – can be micronized in theory, the efficiency of the process and the extent of size reduction can be affected by a material’s chemical and physical characteristics. Plastic materials or those with a high level of water and/or solvents may result in low or negligible size reduction. Materials with a low melting point and/or a tendency to adhesion or crust formation can be addressed with the appropriate engineering solution, such as cryogenic conditions, product contact material, and special geometric adaptation of the milling chamber.
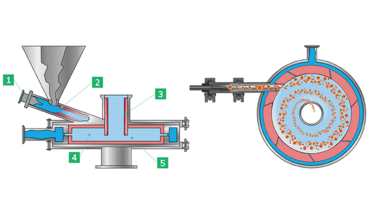
Figure 1: Spiral Jet Mill schema. 1 - Injector nozzle; 2 - Venturi tube; 3 - Upper classification pipe; 4 - Micronization chamber; 5 - Bottom classification pipe.
What challenges does micronization pose?
First, the properties of the micronized material are different from those of non-micronized material; the increased surface area can result in poor flow and cause electrostatic charge to build up. Inadequate flow can cause handling problems in downstream processes, which means that excipients (such as glidants, binders, and lubricants) may be required to improve flow properties. Material can also become more hygroscopic, so storage conditions may require humidity control.
Given the higher specific energy required to reach the desired particle size distribution, there is also a risk that phase changes or side effects could occur, such as conversion to a different crystalline polymorph, dehydration or desolvation, or production of amorphous content.
To avoid these challenges, the solid state of the material needs to be well characterized, meaning the appropriate techniques, such as X-ray powder diffraction (XRPD), differential scanning calorimetry (DSC), dynamic vapor sorption (DVS) or Brunauer-Emmett-Teller (BET) surface area analysis, need to be employed to highlight differences in the material before and after micronization. For example, amorphous content may require additional processing to obtain a stable product prior to formulation. Oftentimes, surface amorphous content rapidly converts back to the crystalline phase under ambient conditions, causing product aggregation or agglomeration, and thus leading to a particle size increase. Short-term physical stability studies can help assess any conversions.
Optimizing working conditions is a sustainable and efficient way to ensure that these challenges are minimized. Here, I offer some best practices:
- Identify all possible critical process parameters, including the variability of the raw material size, water content specifications, and knowledge of solid-state changes.
- Conduct a stress test and a short stability study to assess how the energy applied will affect the solid state of the API, as well as its impact on contingent changes on post-micronization stability, such as particle size increases. It could take around one week and should be factored into the development timeline.
- Perform at least one confirmation run. During the trial duration, ensure that you are assessing the different variables in the process by implementing intensive sampling protocols to help fortify the results you have gathered.
Going further
Particle size reduction technologies – such as micronization – are well-known manufacturing processes with an established track record. They are highly flexible, have the ability to be scaled up, are good for substances with poor thermal stability and are easily applicable to different chemical properties. However, the technique requires a number of resources, including experts and well-equipped facilities. Emerging companies may not have access to these resources or a team with commercial expertise, and even larger companies may not have the appropriate resources. And that’s why most companies choose strategic partners, who can be especially helpful under accelerated timelines with the goal of scaling up.
What is the future of micronization? Although it is an established technique, I think there is still room for improvement. Advances in computing power may help us develop more accurate models to predict gas flow or better study particle behavior. In addition, improved design, automation, and AI could be applied to make the process more predictive and reliable.
Salvatore Mercuri serves as head of Research and Development at Lonza’s site in Monteggio, Switzerland, which is Lonza’s European Center for Excellence for Particle Engineering, specializing in particle size control through micronization. Salvatore’s expertise includes the development of highly potent active pharmaceutical ingredients (HPAPIs) and the formulation of modified-release solid oral dosage forms. He joined Micro-Macinazione SA in 2013, prior to its acquisition by Lonza, and has held positions of increasing authority. Salvatore graduated with a degree in Industrial Pharmacy before earning his PhD in Biopharmaceutics and Pharmacokinetics, both from the University of Parma. He completed postdoctoral work in the Department of Pharmacy at the University of Parma, focusing on modified-release dosage forms.



















