Taking Down a Goliath
Big pharma, vulnerable supply chains, an international religious network…
The story of the Global Pharma Health Fund reads like the plot of a conspiracy novel. But for its developer Richard Jähnke – winner of the 2017 Humanity in Science Award – the reality of fighting counterfeit medicine is far more prosaic. Equipped with his sling – a case full of chemicals, a basic TLC test, and a training manual – his aim is simple: to help spot fakes before they reach consumers.

Counterfeit medicines are a problem of epidemic proportions, particularly in resource-poor countries. In 2000, the World Health Organization (WHO) reported (1) that of all poor quality and substandard counterfeit falsified products, 80 percent do not contain any active ingredients, do not contain enough active ingredient, or even contain the wrong ingredients – leaving patients with drugs that are at best ineffective, and at worst potentially fatal. Complex supply chains provide too many opportunities for adulteration, while understocked labs and expensive analysis equipment only compound the problem of detection, particularly in countries with limited financial resources and patchy power supplies. What is needed is a simple, low-cost and transportable analytical toolkit to protect the supply chain – and, ultimately, consumers.
A tough challenge, perhaps. But Richard Jähnke, as part of the Global Pharma Health Fund (GPHF, based in Frankfurt – a charitable intiative led by Merck, Germany) has spent the last 20 years working with a small team of chemists and pharmacists from universities and the pharmaceutical industry in Germany to develop and deliver a life-saving solution. Its name? The “GPHF-Minilab.”


A bicycle built for… chemical analysis
How did the Minilab come into being? Jähnke, a former Principal Scientist at Beecham Pharmaceuticals, UK, and Business Development Manager at the German branch of PCI Pharmaceutical Services, Philadelphia, recalls his bold (and wonderfully naïve) declaration on finishing his pharmacy degree in Bonn: “I was talking to friends about what we were going to do with our lives. I said I wanted to go into international health development work – and that I would try to make sure the pharmaceutical industry was paying for it. It was a big statement at that time!” In search of a worthy venture after being awarded a Master of Business Administration (and brushing up on his English language skills), a fortuitous meeting with the GPHF – and a subsequent discussion with the WHO – led to the birth of the Minilab project.
The WHO provided a clear but also challenging specification for the project: a test kit for rapid medicine verification and quality monitoring in the field, for low- and middle-income countries. The kit needed to be transportable, reliable, affordable, and unsophisticated, allowing people on the ground to monitor medicine quality with minimal training. It needed to be used by health or medicine-supplying facilities, as well as drug supply organizations in the private and public sectors, and in places with little access to fully fledged operational laboratories. But Jähnke is quick to point out that the Minilab was never intended as a laboratory replacement. “We wanted a ‘complement’ to the lab – when it’s not in full working order, or one simply doesn’t exist.” He spent the next two years developing the Minilab.
Jähnke in no way takes full credit for the idea behind the kit, acknowledging the importance of good timing – and the foresight of his colleague Tom Layloff (Senior Environmental Health Advisor at the Partnership for Supply Chain Management), who he dubs the “grandfather” of the Minilab. “In 1985, there was a big WHO conference in Nairobi, about the quality of medicine in sub-Saharan Africa. People were aware of the circulation of fake medicine, and wanted to discuss ideas for improving pharmaceutical supply,” he says. “Lots of observers from the industry were getting involved – but we needed to stop talking and take action. Tom was working for the FDA at that time, and started to develop some simple thin-layer chromatography test methods – but didn’t have an appropriate toolbox and couldn’t get funding for it. It wasn’t the right time. Ten years later, it started to gain momentum.”
Jähnke realized that to be a success, the Minilab must improve upon the accuracy of previous dye-testing methods, yet be cheaper than the HPLC methods used in the lab. “HPLC is the Mercedes Benz of instrumental analysis, but we only needed a bicycle,” Jähnke explains. “In this context, we did not need fully fledged, sophisticated testing – detecting the absence of a drug is relatively easy.” The resulting Minilab uses thin layer chromatography to test for the presence of 90 drugs, and also includes physical tests for degradation or solidification, which can prevent adequate release of the drug and thus render them useless.
As well as working with input provided by the WHO, the team consulted churches and faith-based organizations who are involved in health initiatives in low- and middle-income countries. “Such organizations gave input as to the cultural background, and what would and wouldn’t work,” Jähnke says. “For example, when I came to develop the manual, they told me I needed far more extensive operational procedures than in the British or US pharmacopoeia. On the other hand, I was told the list of materials in the pharmacopoeia is too long; when people have to do a test on, say, amoxicillin, they would normally consult the pharmacopoeia, then run around the lab, identifying the equipment and chemicals needed – of which 50 percent were likely to be missing.” To prevent the problem, the team had to include all the chemicals, reference standards and solvents needed, so that testing could be performed on the spot. “When you order or use a Minilab, what is written in the text can be instantly performed. There’s a starter kit of chemicals, and everything you need to do the job is right there,” says Jähnke.
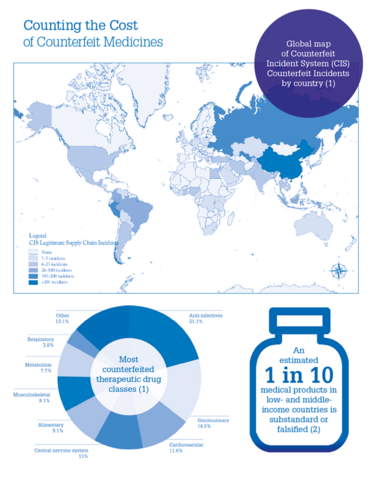
Counting the Cost of Counterfeit Medicines
References
T Mackey et al., “Counterfeit drug penetration into global legitimate medicine supply chains: a global assessment”, Am J Trop Med Hyg, 92 (Suppl 6), 59–67 (2015).
WHO, “1 in 10 medical products in developing countries is substandard or falsified” (2017). Available at: bit.ly/2k82r7m. Accessed January 4, 2018.
Have lab, will travel
From a logistical standpoint, the Minilab comes in a heavy-duty flight case, which contains all the appropriate labware and consumables. The ‘hub’ weighs approximately 25kg, but a starter kit of about 20 boxes of chemicals and solvents is also included in the shipment. Sending scientific equipment to remote regions is a challenge, but Jähnke is proud to note a solid track record in his own supply chain. “We have sent Minilabs to every corner of the world – to the Philippines, to Tanzania, to Ghana – and although they have arrived late in some cases, we’ve never lost one in transit completely,” he says.
The bulk of the Minilabs go to national medicine control labs or public health facilities run by the state. “At the beginning, we focused on the quality of antibacterial and antimalarial medicine. These are public health concerns, so it’s the responsibility of the state to make sure the medicine is accessible and of good quality for people in that country,” says Jähnke. If there is no manufacturing capability in the country itself, the state buys the medicine by tender process, probably cheaply from China or India. Medicine is delivered to central medical stores, then distributed to regional medical stores – and from there goes to general and referral hospitals. Faith-based drug supply can be even more complex.
For African countries in particular, churches have proved to be an invaluable partner in interactions with local communities. “UNICEF and other global tender organizations might order 10 Minilabs for Congo, but they will not tell me precisely where they are going. We procure and send the kits, but are disconnected from their use,” he says. “I find with church groups, there is more of a rapport – I talk to them, I know them, and it’s more transparent.” And though the state might have to answer to its people, faith-based groups answer to a higher power. “They don’t have much money and are quick to spot when they are being cheated. They track fake medicine down even more effectively than the police – because in their eyes, delivering counterfeit medicine with nothing inside is ‘like cheating God’.”
The human factor
Jähnke believes that cooperation with partners has played a crucial part in getting the Minilab in front of the right people and into the hands of those who benefit the most – describing the Minilab as “a success not only of science, but also of public relations.” Recognizing the value of the Minilab, the US pharmacopoeia has helped to market the kit as part of its global health impact program.
“They are very well connected, with access to many governmental labs… but most importantly, they care about the technology,” Jähnke says. The result of such support is few limitations in geographical reach, or in funding. “They were very good at negotiating with the US Agency of International Development to get the funding and, through the Center of Disease Control (CDC), they had access to every embassy. In terms of marketing, when they became involved in the project it was like a hot knife going through butter.”
Working closely with the WHO has also been a real boost to the Minilab team, affording a type of protection that might otherwise have been difficult to attain for a micro enterprise; Jähnke describes the relationship as “a gentleman’s agreement” rather than contractual protection. “They told me that to survive as a small operation, I should follow them as long as I can.”
Jähnke’s own visibility has helped build public trust in the product. “I gave a presentation in Africa, and an audience member said, ‘This is the first time I’ve seen a professor and not a politician!’ They trust me because they can see I have no hidden agenda – I’m not telling them what they want to hear. The most interesting parts of this job go beyond the professional – wherever I go, I travel there as a human being and, ultimately, that’s how you make connections.”
But that’s not to say that he has never caused a stir. The authorities in certain countries have been known to keep a watchful eye on his activities. “One Minister of Health admitted that the state was unable to carry out the testing themselves, but went on to say that it doesn’t mean anyone else is allowed to do it. They reminded me that they were observing me – and that they could have thrown me out of the country at a moment’s notice! But we always find a way around...”
Put to the test – and then further optimized
Jähnke was delighted to have the impact of the Minilab retrospectively confirmed by a WHO report. In a study to identify the scale of the problem of falsified medicine, the WHO checked 100 publications in 88 countries from the last ten years (2), comparing the Minilab with HPLC and other technologies. They checked the reports on 48,000 samples, of which 20,000 were tested by the Minilab – and of these, 1,000 were found to be fake or of extremely poor quality.
“We now know the impact of the Minilab statistically. Case by case, we knew we were doing well, but our claim that the Minilab saves lives has now been backed up by the authority of the WHO. The GPHF is a micro enterprise, so it can feel like David versus Goliath!” The constant observation that goes hand in hand with a higher profile has increased the pressure on the team, however. “We’ve essentially been developing test methods in the public arena, so there is nowhere to hide!”
The Minilab covers a broad spectrum of drugs within the anti-infective arena – the next step is to expand into testing of drugs for non-communicable diseases; for example, cardiovascular, anti-diabetes, and gastrointestinal medicines. Jähnke believes there is room for technical improvement for the Minilab too, such as using a smartphone camera to improve the assay reading, which is currently done by eye. “We want to combine the TLC plate with a final assay reading and interpretation connected to smartphones.”
There is also no shortage of countries still in dire need of the Minilab’s capabilities. “We would like to focus on regions where there are not enough Minilabs available. I’d like to supply more Minilabs for Congo, Cameroon, Chad, Benin, Togo, and the Ivory Coast. If there’s a counterfeit medicine hot spot in the world, it’s Francophone West Africa – whenever we go there, we find something. I would also like to supply countries like Libya, Sudan, Djibuti, Syria and Yemen - but currently, it is just too dangerous.”
Finally, Jähnke would like local workers to take over training on the device. “I have done about 50 training sessions in the past 18 years, and I would love for them to be run independently. I want to empower local people to do the job.”

What’s Inside the Minilab?
- Glassware for sample extraction, preparation, pipetting and spotting
- High performance chromatographic plates
- Developing and detection chambers
- Electronic pocket balance
- UV lamps with different wavelengths
- A hot plate
- Calliper rules
- A full collection of secondary reference standards for 90 active ingredients
- A set of manuals providing simple operation procedures.
A three-point plan
Testing with the Minilab involves three steps:
- A physical inspection scheme of dosage forms and associated packaging material for an early rejection of the more crudely presented counterfeits
- A simple tablet and capsule disintegration test in order to verify label claims on enteric-coating and other modified-release systems
- Easy-to-use thin layer chromatographic tests for a quick check on drug content, thus verifying label claims on potency.
Supplies include sufficient quantities in order to perform about 1,000 assays while ensuring that the total material costs for one test run do not exceed two Euros.https://www.gphf.org/en/minilab/index.html
The Humanity in Science Award
The Humanity in Science Award, presented by The Analytical Scientist (a sister publication of The Medicine Maker), in partnership with KNAUER Wissenschaftliche Geräte GmbH, is an international research prize that recognizes and rewards scientific breakthroughs that can substantially benefit human lives.
For his dedication to developing cheap, simple in-the-field tests (sometimes at the risk of his personal safety), Jähnke won the Humanity in Science Award and a $25,000 prize in October 2017. The Award was presented by Texere Publishing Content Director, Rich Whitworth, at industry partner KNAUER’s 55th anniversary celebration in Berlin, Germany.
The Humanity in Science Award will be presented again in 2018. Keep an eye on the website for more details.
More information can be found at: www.humanityinscienceaward.com
Hold the Line
Fighting fake pharma could be regarded as an overwhelming task, but in Jähnke’s case, persistence (and by his own admission, a little luck) has paid off. “When I finished my degree, I knew what I wanted to do with my life, but I couldn’t gain access to the public health arena. Ten years after my final examination as a pharmacist, I got my chance – and since then, I have followed the Minilab from development to production, to advertisement, to delivery, to training. Twenty years ago, when we were starting the project and talking about counterfeit medicine, not many people wanted to listen. But now it’s discussed everywhere.”
Considering the innumerable challenges, does he ever feel disheartened? “On the contrary – I am filled with gratitude that I got the opportunity to carry out this task. I’ve been to big conferences, with legal factions, public affairs, the consumer power groups, and you wonder how anything is moving – they make it so complicated. But I don’t get ground down by the scale of the task. If I am blocked in one area – I just pop up somewhere else!”
In 2017, Jähnke was “extremely flattered” to win the Humanity in Science Award for the Minilab. “You work all your life in a lab, hoping that maybe you’ve made a difference... But an award like this helps you realize you have had some influence. It’s another part of the story that has drawn the Minilab from the lab and onto the world stage.”
A recent post on Facebook about the GPHF’s detection of counterfeit medicine attracted many memorable and heart-warming comments. For Jähnke, one comment in particular struck a chord, when a fellow pharmacist stated, “It’s the first time I have been proud to be a pharmacist!”
“That’s one of the reasons I do this job,” Jähnke explains. “It’s not just about counterfeit medicine; it’s also about promoting the pharmaceutical profession. It gives us a voice.”
Although the battle against counterfeit medicines is far from over, Jähnke feels content. “It’s overwhelming to still be working on the Minilab 20 years later, when projects these days can be so short-lived. We have survived the test of time. I’ve no plans to retire yet, but when I do, I will feel I have made my mark on Earth.”
For further information, see www.gphf.org
Joanna Cummings is Deputy Editor, The Analytical Scientist, at Texere Publishing.
- E Wondemagenchu, “Counterfeit and substandard drugs in Myanmar and Vietnam”, EDM Research Series No. 29, WHO, Geneva, 1999.
- WHO, “A study on the public health and socioeconomic impact of substandard and falsified medical products” (2017). Available at: bit.ly/2CEpHlO. Accessed 4 January, 2018.
A former library manager and storyteller, I have wanted to write for magazines since I was six years old, when I used to make my own out of foolscap paper and sellotape and distribute them to my family. Since getting my MSc in Publishing, I’ve worked as a freelance writer and content creator for both digital and print, writing on subjects such as fashion, food, tourism, photography – and the history of Roman toilets. Now I can be found working on The Analytical Scientist, finding the ‘human angle’ to cutting-edge science stories.



















