The Big Break for Biosimilars?
The first US biosimilar has been pocketed and the game is on. We bring six experts to the table to discuss the impact on industry dynamics.

Back in April 2006, the first biosimilar in the European Union received marketing authorization – Omnitrope, developed by Sandoz as a biosimilar to Pfizer’s Genotropin (somatropin). At the time, there was no sign of a biosimilars pathway in the US, but everyone anticipated that such products would eventually jump the Atlantic. ‘Eventually’ was nearly nine years later when on March 6, 2015, the FDA officially approved the first US biosimilar – Zarxio (filgrastim-sndz) from Sandoz (1), which is approved for the same indications as Amgen’s Neupogen (filgrastim). Is the US biosimilars market now open for business and ready to start churning out a whole raft of new products? That really depends on who you speak to...
Mixed Reactions
Here’s what major organizations in the US had to say about the approval.
Pharmaceutical Research and Manufacturers of America (PhRMA):
“PhRMA supports a science-based, transparent implementation of the BPCIA biosimilars pathway. In order to meet these goals, we urge the FDA to promptly issue appropriate guidances on key outstanding issues including establishing interchangeability, labeling, and naming of biosimilars products, and to finalize outstanding guidances.” (7)
Biotechnology Industry Organization (BIO):
“It is unfortunate that the lack of publicly available naming guidance resulted in FDA’s assignment of a ‘placeholder’ name for the approved biosimilar. We continue to urge the Administration to issue guidance promptly on this crucial matter, as well as on other biosimilar-related issues.” (8)
Alliance for Safe Biologic Medicines
“We are particularly encouraged by the FDA’s recognition that a biosimilar is a different medication, distinct from its reference product, and that the distinguishable name given to this first biosimilar (filgrastim-sndz) allows healthcare providers to clearly differentiate it from the innovator medicine… One area of concern, however, is in the labeling of Zarxio – the labeling of Zarxio does not state that it is not interchangeable with its reference product, what data were supplied to earn approval is not specified, nor whether or not the product was studied in all the indications for which it was approved.” (9)
American Autoimmune Related Diseases Association
“On behalf of the 50 million Americans living with autoimmune disease (AD), AARDA is concerned that the FDA has approved the first US biosimilar drug without first having published any final standards. The FDA has yet to issue final guidance on a range of issues that will impact patient safety, including interchangeability, naming and indication extrapolation.” (10)
There’s no doubt that Zarxio is a major milestone for biosimilars, but while some groups are hailing it as a triumph, others have highlighted their concerns (see “Mixed Reactions”), particularly as the FDA has not yet issued guidance on some aspects of biosimilars, including interchangeability and naming. Nor has it finalized other drafts of biosimilar guidance. Sandoz also has a number of hurdles to overcome before Zarxio becomes available on the US market. Firstly, there’s that lawsuit from Amgen to deal with.
“As per requirements in the US Biologics Price Competition and Innovation Act of 2009 (BPCIA), biosimilar developers must provide originator companies with six months’ notice before launching,” explains Duncan Emerton, who runs The Biosimilarz Blog (www.biosimilarz.com) and is senior director, syndicated insights & analysis, at FirstWord. “Sandoz argues that it has provided this notice, but Amgen says that notice can only be given on the day of FDA approval. Amgen and Sandoz are currently locked in litigation related to this issue, and Sandoz has committed not to launch until April 10, 2015, or a decision by the court, whichever is earlier.”
The court ruled in Sandoz’s favor on March 19, but Amgen says it will appeal. We won’t go into the finer points of the so-called ‘patent dance’ here (read more at tmm.txp.to/0314/patent-dance) – suffice to say, the path ahead is by no means clear.
Late to the table
There is probably one question that many will be asking – particularly those unfamiliar with US law: why has it taken so long for the US to open its doors to biosimilars? Our timeline shows that the US is well behind Europe, Japan, South Korea and other countries, and in fact, the whole process has taken so long that there is a misconception that some biosimilars are already available.
“Prior to this approval, we had a couple of products on the US market that are considered biosimilars by many analysts. But, they weren’t approved in accordance with a formal biosimilars pathway,” says Joshua Cohen, research associate professor at Tufts Center for the Study of Drug Development. As an example, Emerton adds, “Many people actually believe that Novartis’s Extavia (interferon-beta-1b), a treatment for multiple sclerosis, is a biosimilar of Bayer’s Betaseron. Betaseron and Extavia are just different brand names for the same active ingredient, interferon-beta-1b. Moreover, Extavia wasn’t approved via the US’s 351(k) biosimilar pathway. It’s not a biosimilar.”
“There are a couple of reasons why the US is behind Europe,” explains Mari Serebrov, an analyst with Thomson Reuters and author of the report, ‘Biosimilars: A Global Perspective of a New Market: Opportunities, Threats and Critical Strategies 2014’ (2). “Many of the biologics targeted for biosimilars in the EU had longer patent protection in the US. Also, US laws are different. The FDA didn’t have the authority to develop a biosimilars pathway at all until 2010 when congress passed the BPCIA. Before then, the FDA couldn’t do anything. And once it was passed the FDA had to work out how to take this legal statute and turn it into a pathway.”
Alex Waldron, vice president of commercial operations at EPIRUS Biopharmaceuticals, which focuses on the development and commercialization of biosimilar monoclonal antibodies (mAbs), adds, “Based on legislation that had to be overcome in the US, there were always going to be particular problems. I think nine years was probably a little bit longer than a lot of people were thinking it would take. Everyone is just breathing a collective sigh of relief on the fact that there is now a clearer path of acceptance for these products to make it into the US market.”
And now the first approval is in, it is almost certain that others will follow. Cohen expects to see other biosimilars being approved through the US’s 351(k) biosimilar pathway in due time. Other biosimilars are already under review. “Many consider Zarxio and biosimilars of Neulasta (pegfilgrastim) to be relatively easy cases,” he says. “There is a lot of clinical experience with these products overseas (so fewer safety and efficacy concerns) and physicians will be more familiar with them. But I think that some other biosimilars, such as mAbs, will face more of an uphill battle.”
MAbs are far larger and more molecularly complex than other biological medicines and their clinical properties can be affected by many different factors. Assessing similarity between a biosimilar mAb and its reference product is challenging, but not impossible as biosimilar mAbs have now been approved in the EU and in other countries. Since the US biosimilar pathway is in its early days, it remains to be seen whether FDA regulators will warm to the complexities of biosimilar mAbs. So far, only one biosimilar mAb has been filed with FDA for approval – Celltrion’s Remsima (infliximab), which is a biosimilar of Johnson & Johnson and Merck & Co’s Remicade. Remsima has already been approved in the EU, Japan and Canada, and was due for an FDA advisory committee review in March, but this has been delayed “due to information requests pending with the sponsor of the application” (3).
Global state of play
The first official regulatory framework for biosimilars was created by the European Medicines Agency (EMA) in 2005 with the publication of CHMP/437/04 – an overarching guideline defining the key principles of developing a biosimilar. Generally speaking, the biosimilar concept is applicable to any biological medicine that can be thoroughly characterized. Originally, applicants had to conduct studies to demonstrate that their biosimilar was similar in terms of quality, safety and efficacy to a reference medicine authorized in the European Economic Area (EEA). However, in October 2014 the guideline was revised to introduce the possibility of comparing a biosimilar with a reference product approved outside of the EEA, although it must have been approved by a regulatory authority with “similar rigorous scientific and regulatory standards to those of EMA” (4).
The revision comes into force on April 30, 2015, and also includes other amendments regarding the terminology, principles, and requirements for the posology, route of administration and formulation of biosimilars, based on the experience accumulated since 2005. Biosimilars were also covered in previous legislations (Directive 2001/83/EC and Directive 2004/27/EC), and further quality, clinical and product-class specific guidelines have also been introduced in subsequent years.
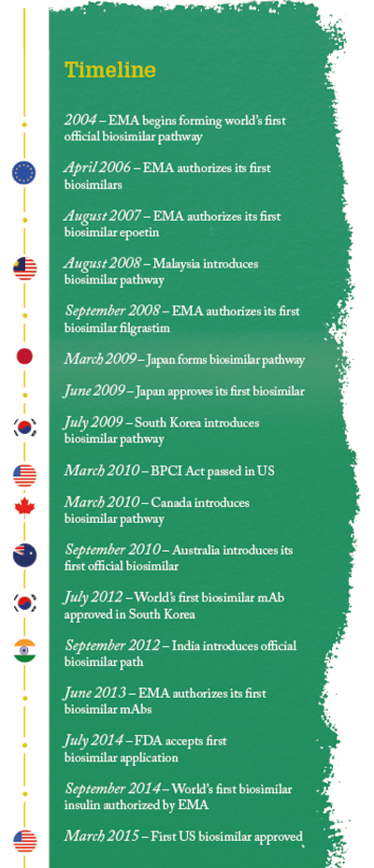
The evolution of regulatory guidance – and the fact that it has been revised so recently – illustrates the youth of the biosimilar market. Nevertheless, biosimilars already have an impressive reach across the globe, with many countries having established some form of regulatory pathway, although like Europe many have also added additional guidelines or made amendments over the years.
“Europe obviously leads the way and has served as a role model for many other countries – mainly because it was the first one out there, plus it’s well thought out,” says Serebrov. “Some countries, like Australia, have adopted the EU model wholesale – when Europe gets a new guideline, Australia adopts it. Other countries take pieces of the European model and tweak it for their market, such as Japan. Once the US model comes out, I think we may see some followers there too. The US can’t follow the European model completely because there are specific criteria that the FDA has to include.”
One of the newest countries to introduce guidelines for biosimilars is China. Draft guidelines were released in November 2014 and finalized in March 2015 (5). Previously, the country had been approving copies for years without similarity studies. “A lot of people are excited about what’s going to happen in China. A lot of biologics haven’t been able to get a foothold in the country because of price. With a true Chinese biosimilar pathway, the industry is expecting a higher standard and also waiting anxiously to see what will happen in terms of exclusivity and patent protection,” says Serebrov. “Brazil is another country of great interest. And some EU and US companies are trying to increase access in Africa and the Middle East through the promise of biologics at affordable prices.” Realizing ‘global health’ is a major benefit of clear biosimilar guidance, and many companies have recognized the potential of new markets. Serebrov adds, “Companies are making innovations in manufacturing processes and development to really lower the price of biosimilars. It’s exciting that we might finally be able to share the hope of these drugs with a larger percentage of people.”
India is another market to watch. “There is potential for huge growth in India,” says Waldron. “One of the reasons EPIRUS launched into the Indian market is because there are relatively low usage levels of biologics, primarily due to the fact that India is a private-pay market. Take rituximab; I believe that when Dr Reddy’s introduced Reditux [a non-comparable biologic of rituximab], it increased the use of rituximab by somewhere between six- and ten-fold over a 5–8 year period. The reason Reditux was able to substantially grow the Indian market was largely driven by its much lower price point – patients who could not have afforded Roche’s Mabthera now had access to treatment.”
As a non-comparable biologic of rituximab, Reditux is not a biosimilar. It was approved in India in 2007 on the basis of a 17-patient open-label study and was not compared to Mabthera. Before India introduced its biosimilars pathway in 2012, several copies of innovator biologics were approved on a case-by-case basis. A number of products have now passed through India’s official biosimilars pathway, although it is difficult to know if all of these were developed in accordance with the pathway’s standards since many will have been in development long before 2012 (2).
Similar... but different
Many companies are jumping onto the biosimilars bandwagon, but hold on Charlie Bucket – it’s no golden ticket. Biosimilars are inherently complex and tiny changes in development can have major implications. As a result, they remain expensive and time-consuming to develop.
“Small-molecule generics are in some ways a bit of a photocopying exercise. Biosimilars are much more difficult. You have to go through exhaustive cell-line characterization, pharmacokinetic work and a clinical study,” says Waldron. “When bringing an innovator molecule to market, drugs are powered to hit a predesignated endpoint in the Phase III clinical trial. Hitting that endpoint is extremely difficult – the challenge for biosimilars is that not only do you have to hit the same clinical Phase III primary endpoints, but you must also show ‘similar’ results to the innovator in terms of pharmacokinetics and pharmacodynamics. Some regulations allow for slight improvements in some areas but if you are too much better then you become a biobetter, which leads to more regulatory hurdles. And obviously you can’t do worse because then you would be clinically ineffective!”
Emerton adds, “The biggest challenge for biosimilars is creating the right economic environment, so that all of the key stakeholders involved in prescribing, using and paying for biosimilars are incentivized to use them.”
Biosimilars do not offer the same cost savings as generics. Typically, biosimilars offer price reductions of 20 to 30 percent depending on the market, whereas generics can offer 70 to 90 percent. “In some markets, biosimilars are much cheaper but in others they can be almost the same price as an innovator’s drug. Some innovators have also dropped their prices to match the biosimilar. With this model, you have a biosimilar that is new to the market, having to build a brand, recoup the cost of development, and competing head to head with a well-known innovator that’s been in the market for years,” says Serebrov.
Although that’s potentially bad news for new companies launching biosimilars and looking for greater market share, there is an upside for patients: lower priced drugs. Competition means that innovators can’t just arbitrarily raise the price every year.
“Biosimilars are targeting the blockbuster biologics – the cash cows,” says Serebrov, “and this is bringing prices down and expanding use of biological medicines to countries and patients that previously couldn’t afford them.”
The American game
Although the US is late to the biosimilars party, it does have one advantage: it has been able to benefit from all the lessons learned over the past nine years. Emerton, however, believes that growth in the US will still be slow initially. “More in-market clinical data has been generated in markets outside of the US and I believe that other markets are more open to the biosimilars concept. That’s not to say that the US won’t get there eventually, but at the moment I don’t see physicians embracing biosimilars in the US,” he says. “Payers, however, are a different story. I’m currently writing a report on US payer perceptions and views on biosimilars, and what’s come across quite strongly is that payers can’t wait to get their hands on biosimilars. They see them as a much-needed safety valve that they can use to ease the pressure in other areas of the health system.”
Meanwhile, Serebrov thinks that the US market may actually open up a lot faster. Although no guidance has yet been issued by FDA on the matter, she believes that interchangeability will accelerate market acceptance.
Race to the Top
By Erwin A. Blackstone and Joseph P. Fuhr Jr.
Economics is based on incentives. People and businesses respond to incentives and when it comes to biosimilars, the incentives seem to be aligning for considerable competition. Although each individual biologic market is unique, some general characteristics exist concerning biosimilar markets. Many billion-dollar biologics are losing or will soon lose patent protection – and the impending opening of the US market, the largest biologic market, will provide additional incentives for biosimilar entry. Furthermore, the fact that biosimilars are often developed by large pharma companies that can withstand the uncertainty and competitive pressures of the market will ensure a competitive race to the top (or bottom).
On the other hand, biosimilar entry entails substantial risk. R&D costs are high and incurred long before the product is marketed. There are also manufacturing, promotional, legal and regulatory costs. In Europe, biosimilars have had modest (or mixed) success because there has been little financial incentive for stakeholders to opt for lower priced biosimilar products. The German government, however, has an incentive system that encourages the use of biosimilars, which has increased uptake. Interestingly, the EU generic market is not as strong as the US, so it’s difficult to predict outcomes. Financial incentives, particularly through pricing, will determine the development of the biosimilar market. In any event, the high price of biologics means that pricing pressures are likely to become stronger.
It also is important to note that the primary policy objective of biosimilars is to increase consumer welfare. Thus, the market share of biosimilars is not a fully informative metric. The relevant welfare benchmark is not the price of the biosimilar relative to the originator, but the comparison price before competition.
Whatever happens, patients should be the final winners, with lower prices and greater access to lifesaving drugs.
Erwin A. Blackstone is professor of economics at Temple University. Joseph P. Fuhr Jr.is professor of economics at Widener University.
To be approved as an interchangeable in the US, the FDA has said a biosimilar must be able to demonstrate the same clinical effect in any given patient. Biosimilars not approved as interchangeables would have to be specifically prescribed by name. Some US states have already enacted biosimilar substitution legislation that will allow pharmacies to automatically substitute an FDA-approved interchangeable for a prescribed innovator.
“If automatic substitution is allowed, we could see faster uptake and biosimilar manufacturers wouldn’t have to promote and market those products that are approved as interchangeables,” says Serebrov. “Automatic is the key word here; there are other countries that allow substitution at the beginning of treatment but once the patient starts on a treatment they tend not to be switched to another version, whether it is innovative or not. In the US – although we don’t have the guidance yet – I think that we could potentially have a more interchangeable system where a patient can be switched back and forth more easily between an interchangeable and the innovator.”
But with interchangeability comes concerns. What happens when there is more than one interchangeable on the market for a given reference product? “There are concerns about how realistic interchangeability is. Will your biosimilar truly be interchangeable with the competition? Or will you have to do switching trials with every single one of them on the market? We won’t know until the FDA guidance comes out,” says Serebrov.
“Payers will likely place biosimilars in lower cost-sharing tiers on the formulary to try to boost their use and reap cost savings. They may sign rebate deals with biosimilar manufacturers that aim to increase their market share, but ultimately, payers will want to have therapeutic interchangeability established, not just biosimilarity. This will be a major challenge, as therapeutic interchangeability is harder to establish than biosimilarity,” adds Cohen.
The naming of biosimilars is a hot topic in the US, with pharmacovigilance issues raising particular concerns, as explored in a previous issue of The Medicine Maker (6). “Should a biosimilar have the same International Nonproprietary Name (INN) as the reference product?” asks Emerton. “In the case of Zarxio, the FDA has given it the temporary INN of ‘filgrastim-sndz’. By going with this temporary naming convention, the FDA has created some confusion and raised the specter that distinguishable names for all biosimilars will become the norm in the US. As I see it, this is a victory for the originator companies, which have lobbied against the use of identical INNs for biosimilars. It remains to be seen if the FDA allows interchangeable biosimilars to have the same INN as the brand, so as to facilitate substitution. Much uncertainty still remains.”
The naming issue isn’t limited to the US. “In Japan, they name biosimilars Biosimilar 1, Biosimilar 2 and Biosimilar 3. And yet there could be two products marketed as Biosimilar 1. For instance, partners Fuji Pharma and Mochida Pharmaceutical each market Filgrastim Biosimilar 1, while partners Nippon Kayaku and Teva Pharma each market Filgrastim Biosimilar 2. I don’t know how that will play out in the long term!” says Serebrov.
Waldron also highlights another hurdle for biosimilar medicine makers. “I think that one of the biggest challenges for biosimilar manufacturers looking to break into the US is the legal landscape in terms of intellectual property. It’s fantastic to have large companies like Sandoz and Celltrion blazing a trail because they will be a solid bellwether for how the patent situation is going to evolve,” he says. “In the US, the major patent is well publicized, but there is no mechanism at this point to firmly establish all patents associated with biologics. I expect to see pop-up or surprise patents coming up last minute to challenge some of these molecules coming in.”
Similarity breeds contempt?
Much will be learned about the potential future of US biosimilars in the coming months as Sandoz and Amgen settle their arguments and Zarxio enters the market. “Who will be the market leaders in the long run – large or small companies?” asks Serebrov. “Sandoz is the pioneer and has the largest share of the global biosimilars market but then you also have Hospira and Teva. And then there’s Celltrion with its biosimilar mAbs. There are so many other companies that are close to launching their first biosimilar. Some of them are pharma giants and some are start-ups, and they’re all following different models so it will be interesting to see which ones are still there ten years from now. We’re also seeing a lot of contract manufacturing companies jumping into the space. In emerging markets, where they haven’t previously had a market for biologics, they’re looking to biosimilars to jumpstart the industry; Brazil, Russia, and South Korea are all getting government help because biosimilars are seen as a big driver for the economy.”
Waldron agrees that smaller companies are in with a fighting chance. “Large biotechs and pharma companies are looking to leverage their legacy of producing drugs. They’re looking to put their giant global key into the lock of biosimilars and assume that it’s going to work in exactly the same way that it’s worked with every other biologic that they have brought to market. For biosimilars to become really profitable – and to get them to market quickly – you have to use today’s technologies and you have to be flexible and able to approach each region on a market-by-market basis. Smaller, newer companies don’t have a legacy, which means that they are always looking forward at new, innovative business models, never backwards.”
But no matter the size, all companies looking for a piece of the biosimilars pie must be prepared for the long haul. Uptake in Europe shows that growth can be slow, and even if the US does embrace biosimilars, it’s still very early days. Sererbov says, “Companies won’t be able to grab a huge market share within a year or two. And there’s a lot of educating to be done to tell patients what a biosimilar is and why it’s safe.”
It’s not just the companies making biosimilars who will have to adapt as the market expands – Serebrov believes that the rise of biosimilars may be a shot in the arm for innovator companies too: “This will really push innovators to do more serious innovation, not just a tweak for a longer acting drug – they will truly strive for innovation. And that’s a challenge for the biosimilars producers, who will need to find new ways to prove why you should buy their product. Does anyone want to take yesterday’s drug when there is something new out there?”
Top Six Biosimilar Misconceptions
... according to Duncan Emerton
- Biosimilars aren’t as safe as the reference products
- The quality of biosimilar products is lower compared to the reference products
- Biosimilar companies aren’t able to undercut branded biopharma companies on price
- Biosimilar companies don’t have the capacity to make enough product
- Biologics are too complex to copy
- Products approved without head-to-head comparison with the reference product, including non-clinical and clinical studies, are biosimilars
Gain more insight from Duncan in the Biosimilar Index: Tracking the Biosimilar Development Landscape (11).
- FDA, “FDA approves first biosimilar product Zarxio” (March 2015). www.fda.gov
- M. Serebrov, “BioWorld Biosimilars: A Global Perspective of a New Market: Opportunities, Threats and Critical Strategies 2014,” (Thomson Reuters, 2014). www.bioworld.com
- FDA, “POSTPONED: March 17, 2015: Arthritis Advisory Committee Meeting Announcement”(February 2015). www.fda.gov
- EMA, “Guideline on Similar Biological Medicinal Products (October, 2014). www.ema.europa.eu
- BioCentury, “China Issues Guidance on Biosimilars,” (March, 2015). www.biocentury.com
- C. Akers, “Safety First - Sizing Up Biologics Side Effects”, The Medicine Maker 3, 35 (2014). themedicinemaker.com/issues/looking-out-for-children/safety-first-sizing-up-biologics-side-effects/
- PhRMA, “PhRMA Statement on First FDA Approval of A Biosimilar Product,” (March 2015). www.phrma.org
- BIO, “BIO Statement on First FDA Approval of a Biosimilar Product,” (March 2015). www.bio.org
- ASBM, “ASBM Commends FDA for Approval, Clear Naming of First Biosimilar,” (March 2015). www.safebiologics.org
- AARDA, “American Autoimmune Related Diseases Association’s Statement On FDA’s Approval Of First U.S. Biosimilar Drug,” (March 2015). www.aarda.org
- Biosimilar Index: Tracking the Biosimilar Development Landscape (FirstWord, 2015). www.fwreports.com

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















