The Crystal Maze
Unexpected hydrates of an API can cause a frustrating detour in the drug development pathway – the right analysis (and expertise) can help manufacturers find their way
David Pearson | | Longer Read
Despite having modern modeling software, cloud-based computing, and an arsenal of analytical protocols at their disposal, predicting the crystalline nature of an API in its solid state remains a trying task for pharmaceutical manufacturers. This is particularly true of hydrates – APIs whose crystal structure contains water molecules. Up to 75 percent of all pharmaceutical compounds form hydrates during the manufacturing process, affecting many of the physicochemical properties of an active ingredient (1).
This is a sticking point for companies wanting to move their API through the clinical pipeline as quickly as possible as it challenges their ability to discern the overall bioavailability, physicochemical properties and intellectual property position of their material early in the development process. To help overcome these hurdles, larger pharmaceutical companies are increasingly turning to CDMOs to perform solid-state analyses in expert laboratories. Valued in excess of $150 million, CDMOs represent over 50 percent of the total market share for solid-state services (2).
Companies should perform comprehensive experimental screening of materials using a variety of solvents, experimental conditions, and processes, along with all the associated analytical techniques. Ultimately, you are looking for a robust solid form of a molecule that allows high-quality materials to be consistently delivered.
But inadvertent changes to the solid form of an API between batches can still occur. So how can companies effectively manage the variability in their manufacturing processes? Identifying and understanding the formation of alternative structures early on can reduce potential risks.
Hydrates: putting development at risk
Unlike solvated forms of an API, which contain organic molecules (such as ethanol) in their crystal lattice structure, hydrate formation relies on water and is not easily recognized during screening. As the vapor pressure of water is above zero, hydration and rehydration can occur in ambient conditions such as storage. If a drug were dosed in an anhydrous form that converts during storage or in the body to a lower-solubility hydrated form, for example, it may affect the observed solubility and dissolution of the API, making in vitro/ in vivo correlation more difficult.
If the potential to form hydrates is flagged to the development team early on, they can take steps to mitigate the risk. What does this look like in practice? We used polymorph screening to examine the difference in four different crystal forms of an API obtained from organic solvent and solvent/water mixtures.
The various solid forms included:
- Form 1 – anhydrous racemate and the desired form of the API
- Form 2 – a hydrated racemate
- Form 3 – anhydrous conglomerate
- Form 4 – a hydrated conglomerate
The rate at which these hydrated forms dehydrated varied massively – from several minutes under ambient conditions, to several days at 40°C in a stability oven (Figure 1).
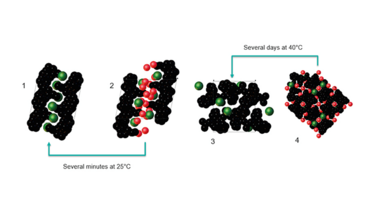
Figure 1. Different structures of API – different dehydration rates.
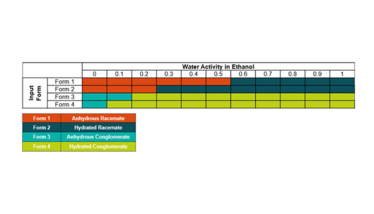
Table 1. Hydrate mapping of the four API forms.
Initial data indicated that there were two anhydrous and two hydrated forms (with the same amount of water observed in the two hydrated forms). Only detailed analysis by X-ray powder diffraction (XRPD), single-crystal X-ray diffraction, dynamic vapor sorption (DVS), and thermal analysis showed that the two hydrated forms were not true polymorphs of each other. This allowed the targeting of the desired form.
Hydrate mapping, in which the water activity in organic solvent systems is systematically altered using varying volumes of water, is a powerful technique to understand the hydrate formulation in solvent-based systems. While DVS and variable humidity XRPD can provide a wealth of information on critical water activities and kinetics, a more practical method is solvent-based and closely related to real-world scale-up. Table 1 shows the results from the hydrate mapping for this particular API and its four forms.
We recognized that there were a variety of ways of obtaining metastable zone width measurements, or solubility data, using tools such as focused beam reflectance measurement (FBRM), turbidity probes, Crystal16 and the recent BlazeMetrics probes. In this case, a cooling crystallization was used and the metastable zone width measured in a variety of different solvent systems. This data was also used to give an early indication of particle size, morphology, and other properties. The ethanol-heptane system gave the widest metastable zone width, which allowed greater flexibility over the seeding point and de-supersaturation rates. The crystal form produced from these experiments was also checked and found to be the desired form 1, even without the use of seeds.
Once the solvent system was identified – and in this case, ethanol:heptane looked ideal – crystallization was optimized with an in situ, real-time analysis of the process. Using a BlazeMetrics probe, particles were monitored as they were produced and this provided a wealth of information on the formation, growth, morphology, and size distribution. The large particles obtained filtered very rapidly, dried easily, and ultimately gave a high-quality product and a process that could easily scale to a vessel size in excess of 1,000 liters.
Online Raman spectroscopy was not required in this case because the use of a non-aqueous solvent system reduced the risk of hydrate formation. However, it can be used to observe the formation of an undesired crystal form or, more importantly, confirm that there is no change in form occurring within the vessel during the crystallization process.
Importantly, our work shows how an understanding of the different crystal forms informs the selection of appropriate protocols. This will ultimately help accelerate the development of an API, reduce risk in the development and manufacture of both the drug substance and drug product, as well as adding intellectual property to the portfolio.
- J Christholm et al., "Knowledge-based approaches to crystal design," Cryst Eng Comm, 8,11 (2006).
- Cambrex Marketing & Intelligence; (2019)



















