The Hype, Hope and Reality of Personalization
Personalized cell-based therapies have been hyped as the future of medicine, but are current manufacturing technologies up to the task?
Behzad Mahdavi, Uwe Gottschalks, Nuala Trainor, Tim Smith |

Cell-based therapies have been celebrated as a new approach to healthcare because of their potential to treat a wide variety of unmet clinical needs – from cancer to arthritis to spinal cord injury. Cell therapy involves administering living cells to a patient with the aim of replacing missing or damaged cell types, potentially treating the underlying cause of a disease. Some cell therapies have already been approved by regulators and with further research taking place, we can expect to see many more in the future.
The big question is, how do we ensure that these therapies, especially personal cell-based therapies (PCBT), can be commercially manufactured to Good Manufacturing Practices standards at an acceptable cost and quality – and in the right quantities? After all, PCBTs are radically different to both classic small-molecule drugs and even biopharmaceuticals.
Cell-based products require expansion or manipulation in the lab using advanced cell culture and tissue engineering platforms. Cell therapies can be segmented into two groups: autologous and allogeneic, depending on the sourcing of the cells. Autologous cell therapy products are produced from the patient’s own cells, whereas donor cells are used for allogeneic cell therapy. Partly because of the sourcing differences, autologous and allogeneic cell therapies face different manufacturing challenges, and therefore require different strategies to enable translation into commercially accessible and affordable medicines. The major advantage of the autologous approach is immunological compatibility; using a patient’s own cells obviously eliminates immune-rejection.
A subset of allogeneic therapies are “patient-matched,” which means that, although donor cells are used, the final product is custom made for one specific patient at a time – in very small batches. From a manufacturing perspective, this means that each patient requires their own batch. In this article, we focus on PCBT, which includes both autologous and patient-matched allogeneic therapies (see Figure 1) for more than ‘minimally manipulated’ cells – an FDA term.
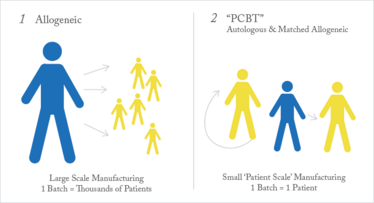
Figure 1. Patient-scale manufacturing.
The ideal long-term solution, of course, would be a universal allogeneic approach where we could create perfectly matched therapies with no danger of immune-rejection, but the majority of candidates in clinical pipelines at the moment – and most likely to hit the market in the near future – are PCBTs. In the following sections, we offer an overview of PCBT manufacturing and operational obstacles and the manufacturing strategy to overcome these challenges.
The product is the process
When working with PCBTs – or any cell-based therapies – the process and the product cannot be dissociated. As with the early stage of development for recombinant proteins and monoclonal antibodies, cell therapy products are defined by their manufacturing processes and equipment, as well as the facility in which these products are made.
The ‘source material’ of autologous cell therapies is one challenge as the patient can be considered as one of the raw materials. The wide range of ages and physical conditions of cell donors introduces highly variable inputs to the manufacturing process in terms of a healthy number of cells to start the expansion, as well as their proliferation capacity. For example, in T cell expansion, the rate of growth of specific populations may be influenced by the disease state or age of the patient. To help ensure the consistency and quality of the final product, you can use automated systems that have built in reactive biofeedback. Reactive biofeedback uses real-time monitoring (for pH, O2, glucose etc.) and software with pre-defined protocols that can adjust and keep critical parameters influencing cell growth within certain predefined ranges to help ensure that the end product has narrower release criteria.
Systems like this are available, but they cannot all work with variable source material or different units of operations. When investing in new equipment you should consider systems that have been specifically designed for cell therapy work and able to scale out to commercial production. In this regard, there is a need to employ automated systems that are flexible and able to accommodate different processes with different cell types and cell culture methods if you want to develop a universal manufacturing platform for PCBT. In other words, the system should be adaptable to fit and reflect the details of the process, rather than require that the process be adapted to fit to the system. Such a characteristic preserves the uniqueness of each process/product, which in turn is useful for preserving the intellectual property component of the therapy (which we all know is an incentive for developers).
Because the manufacture of PCBTs involves many small batches, large processing equipment is not suitable. Instead, many small-scale systems and bioreactors are needed – which can become expensive. Many see automation as the ultimate solution for manufacture of PCBT as, in general, it helps to bring costs under control and facilitates the operations. However, the cost structure of the manufacture of PCBTs is complex. The main costs are associated with labor, which can be handled by automation, but there is also the cost of the clean room space, which is defined by the overall footprint of the equipment and process. Each batch of a PCBT requires its own cleanroom space, which is something that many manufacturers forget to consider when investing in commercial, automated cell therapy systems. Automation can certainly minimize the number of manual cell-handling tasks to provide greater reproducibility, traceability and overall specification compliance, but it does not always offer overall space efficiency. Instead, a preferred economical solution would be to select systems that have been specifically designed to be compact – and preferably stackable. Stackability cannot be exploited if each batch requires its own cleanroom space, but there is another solution: a closed manufacturing solution. With more cell-based therapies receiving greater attention in industry, equipment more suited for the commercial manufacture of PCBTs is coming onto the market, including fully automated, stackable and closed systems where you can fit eight closed systems (translating to eight batches of product) in one square meter. The closed system is a very important feature as it minimizes and eliminates the potential risk of contamination between different unit operations. A closed system also allows for manufacturing to occur in a lower class room with less requirements for environmental monitoring and control.
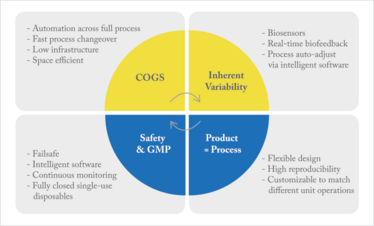
Figure 2. Overcoming the challenges of manufacturing personal cell-based therapies.
Contamination control
When discussing cell therapies – or any biological medicines – the potential for contamination is always a concern. For PCBTs, contamination could come from the improper handling of cells during splitting, seeding and changing media – or from any mistake during processing. Cells are very sensitive and you can never be too careful. As it is not possible to sterilize the output of a cell manufacturing process in ways similar to other medical devices, comprehensive cleaning and handling protocols are required to minimize the risk of contamination. Furthermore, in-depth quality control tests must be performed to verify that no contamination occurred. Whatever the cause of contamination, the most important consequence is that the batch will be a loss. In most cases, a lost batch of a biological drug means lost profits, but the consequences are far worse for PCBTs because production is not easy to restart because of the length of time required; in many cases, the patients receiving PCBTs are very sick and cannot afford to wait or restart a process.
Behzad Mahdavi, is VP of Strategic Innovation & Alliances, and Uwe Gottschalks is Chief Technology Officer at Lonza. Nuala Trainor is Director of Biological Programs, and Tim Smith is Chief Executive Officer at Octane Biotech.
To reduce contamination risk, cell manipulation and culture process should be conducted in a fully pre-steriled closed system. When the tissue biopsy or cells are collected from the donor at the clinic, they should be introduced via the hood into the clean system. All subsequent steps of manufacture then take place within the closed system, with one closed-system cell manipulation unit being used for each patient to help eliminate cross-contamination. When dealing with any kind of cell therapy – or any donor biological material – it is standard practice to keep all samples isolated from one another. This actually becomes far more complicated than you might naturally assume when you are in a commercial setting because you could be dealing with more than 10,000 patients per year. When you have that many batches, human error can happen more frequently, particularly if you are managing samples that are not produced in single-use technologies. We also recommend using patient-specific reagents for each cell culture unit, which can be achieved economically and automatically with single-use cell culture units and fluid-contacting components available as a pre-sterilized system that can perform all processing events. Such systems reduce the risk of patient-to-patient contamination to zero – and also avoid validation of cleaning processes for non-disposable equipment (and the associated cleaning costs). With disposable cell culture units, each disposable component can be tagged to a specific patient, which aids in traceability and secures the logistic chain.
Despite best efforts across the industry, most of the current platforms and solutions for the manufacture of PCBTs don’t fulfill all the necessary requirements. New technologies, however, are emerging that will enable manufacturers to build safer, more efficient, processes that overcome some of the manufacturing challenges, providing that the manufacturer has a good knowledge of critical process controls. This type of approach can also fit with both centralized and decentralized production models, in the sense that manufacturing production can be centralized in one manufacturing plant in an efficient and economical way, with the products shipped to different point of the care facilities. Or you can use a decentralized model where different smaller manufacturing units are used at the point of care.
Top Tips for Commercial Cell-Therapy Manufacture
- Systems should be automated and space efficient.
- Employ automation systems in the early stages of new therapeutic development to facilitate scale out later on.
- Systems should be flexible and capable of being customized to suit the process; you shouldn’t modify your process to fit to systems.
- Each batch should use a personalized bioreactor.
- Single-use technology solves cleaning validation issues and reduces risk.
- All pathways and fluid circulation should take place in a closed system.
- Use intelligent software and reactive biofeedback to boost automation and to accommodate for natural variations in the starting patient’s cell.
- Automated processing should be able to generate comprehensive electronic batch records that can be merged with production management software.
Despite the challenges posed, personalized cell-based therapies are an exciting alternative to small molecule and biologics – and really could be the future of medicine. The (bio)pharma industry’s perspective on regenerative medicine is maturing and shifting from the cautious role of the observer and venture-capitalist to a more focused approach with active research and clinical development teams. In particular, the industry has seen impressive early clinical results from chimeric antigen receptors (produced by genetically engineering T cells to produce receptors that allow T cells to recognize antigens on tumor cells), which is exciting and also fueling interest. The demand for such therapies could be extraordinary and hopefully new game-changing technologies will help to complete the missing piece of the puzzle for a successful commercial PBCT to meet clinical demand and demonstrate rigorous efficacy.
Manufacturers must learn how to grow cells on a commercial-scale for each patient in a cost effective way, while meeting ever-evolving regulatory and quality requirements. Examining manufacturing strategies and closely evaluating the latest technology is the only way to transform promising products into commercially successful – and affordable – medicines of the future.
VP of Strategic Innovation & Alliance. Uwe Gottschalks, Chief Technology Officer both at Lonza
Chief Technology Officer both at Lonza
Director of Biological Progams
CEO at Octance Biotech



















