Top Tips to Optimize Downstream Processes
From resins to buffers to single-use technologies – there are many opportunities to improve downstream processes
Nandu Deorkar, Jungmin Oh, Pranav Vengsarkar, Jonathan Fura | | 6 min read | Practical
Emerging treatments, including cell and gene therapies, are exciting and are certainly starting to expand pipelines, however, traditional biologics; monoclonal antibodies, still dominate the world of biopharma. Research has shown that the clinical pipeline of antibody therapeutics grew by 30 percent over the past year (1) – excluding COVID-19 antibody therapies – highlighting the importance of these treatments and the need for their efficient production.
Given that 60–80 percent of mAb production costs can be attributed to downstream processing (2), removing downstream bottlenecks, and improving yields will continue to be an important priority for mAbs manufacturers – especially amidst rising demand. Below, we offer a few suggestions.
Considering resins and buffers
In the capture step, protein A is the most widely used resin. Protein A is simple to implement as a standard purification process and holds a strong regulatory track record (3); however, the costs of the resin are substantial, making it important to optimize the process to maximize cost and efficiency. A key consideration in process optimization is understanding the role dynamic binding capacities (DBC) plays in overall protein A performance. Use of a resin with higher DBC can improve capture step productivity while maintaining column sizes and minimizing facility modification – especially when it comes to high titer cell culture processes.
To prove the point, we performed a simulation using BioSolve software, calculating the number of bind/elute cycles, process time, and volumes of buffer required for a 2000 L bioreactor batch. We looked at three model resins with DBCs ranging from 30 g/L–65 g/L. Assumptions made for the calculations are summarized in Table 1. We maintained column size at 68.6 L for 2000 L cell culture reactor with 5 g/L titer value. We evaluated process productivity based on the number of cycles required per batch as well as process time.
Table 1. Simulation process parameters
What did we find? Higher DBC resins significantly reduce the number of cycles and total downstream processing time (see Table 2 and Figure 1). Notably, by reducing the number of cycles, one can also reduce operational risks and per-cycle costs for labor and consumables.
Table 2.
Similarly, a lower volume of buffer consumption not only reduces raw material cost, but also buffer preparation time, buffer tank size, and method of preparation. In our model, Resin C reduced total buffer consumption by approximately 40 and 30 percent when compared to Resin A and B, respectively.
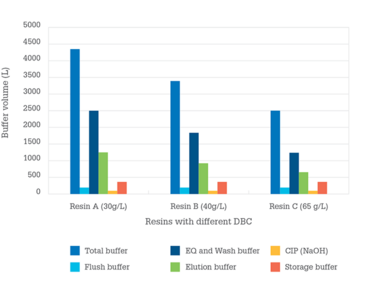
Figure 1. Buffer consumption of three protein A resins with different dynamic binding capacity (DBC) for processing of one 2000L bioreactor batch
Creating buffers in-house is a well-established method suitable for manufacturing large volumes, however, preparation of buffers often involves utilities and resources, such as Water for Injection (WFI), which may be constrained due to demand in other systems such as clean-in-place or other process lines. Further, the sheer number and volume of buffer solutions required for the entire downstream purification process may cause scheduling issues for the buffer prep team trying to meet the demands of the production schedule. Reduced buffer solution requirements offer additional flexibility as these operations require significant infrastructure, including warehouse space for holding raw materials prior to their use, a weighing and dispensing area for raw materials, and space to store the prepared solutions which are often stored in corridors due to lack of space. In addition, the stainless-steel tanks themselves can require a considerable footprint in the facility and frequently experience corrosion issues due to the caustic nature and high chloride content of commonly used buffers.
New developments in single-use technology have added flexibility in buffer preparation methods, allowing small- and medium-scale facilities to move to single-use tanks for buffer preparation. This has enabled faster changeovers in buffer preparation, saving both time and cost in manufacturing processes (4). Single-use fluid handling systems can help reduce bottlenecks, particularly in cell therapy manufacturing where downstream processing is often slowed by lack of the suitable closed manufacturing systems. The closed, automated systems that are available are often unsuitable for large volumes of allogeneic cell therapies. If a biomanufacturer uses single-use equipment, a reputable supplier with a multiple-source supply chain is key to avoid disruptions.
A hybrid approach
Combining both in-house systems and outsourced buffers in a hybrid approach can help streamline downstream purification unit operations. Moreover, in-line dilution (ILD) systems can improve the efficiency of critical buffer component production.
- Clean-in-place solutions: Usually a fixed normality of NaOH, it can be prepared in-house using concentrate or purchased as a 1X concentration thanks to the smaller volumes needed, lowering safety concerns.
- Storage buffer: Due to low consistent volumes typically required (irrespective of resin DBC), storage buffers, such as 20% ethanol, can be managed in-house in the same way as the cleaning buffer.
- Equilibration and wash buffers: Volumes of these buffers (for example, 1X PBS or 50 mM Tris, pH 7) significantly decrease with an increase in resin DBC, as shown in Figure 1. Whether these buffers are prepared using in-house or single-use systems, high volumes can cause several operation challenges. When preparing these buffers, inline dilution (ILD) systems using multicomponent concentrates (for example, 10X PBS) can provide operational advantages. For example, ILD can help minimize facility footprint, reduce raw material management, and increase availability of buffer on demand.
- Elution buffers: Use of these buffers (for example, 0.1M acetate buffer, pH 3.4) can also be streamlined through the use of an in-line dilution system.
Workflow improvements in buffer preparation
Broadly, there are three options for buffer prep system/process in downstream purification:
- Single-use buffer prep reactors or chemical hydration in fixed stainless-steel tanks
- Multicomponent buffer concentrates with in-line dilution or single component stocks with buffer stock blending
- Ready-to-use cGMP 1X buffers
BioPhorum Operations Group (BPOG) and other industry organizations have offered insight into how buffer stock blending and in-line dilution generate overall improvements across unit operations (4, 5, 6). Choosing the right option will usually depend on an economic analysis of several factors, including scale, batches of drug produced per year, raw materials required, and other site attributes.
Table 3 offers workflow improvements for each of the three options.
Table 3.
The flexibility and productivity of the mAb capture process step can be improved by using high DBC resins along with optimal buffer management. High-capacity resin decreases process time by allowing less numbers of cycles required per batch — saving cost, mitigating risk, and reducing labor costs.
In addition, implementing a high DBC resin decreases the volume of process buffers significantly, which allows the flexibility to adopt different buffer preparation processes based on facility requirements. Each facility and downstream process has unique requirements and bottlenecks, so having flexible process optimization options is important.
As innovative biologic treatments continue to emerge, manufacturers will almost certainly face even more hurdles – but, in every situation, the development of highly efficient, high yield manufacturing processes will be a key factor for success.
- H Kaplon et al., MAbs 14 (2022). DOI: 10.1080/19420862.2021.2014296
- BioProcess International, “Key challenges and potential solutions for optimizing downstream bioprocessing production,” (2019). Available at https://bit.ly/3GlBxzi
- T Pabst, J Thai, A Hunter, Journal of Chromatography A 1554, 45-60 (2018). DOI: 10.1016/j.chroma.2018.03.060
- P Vengsarkar, N Deorkar, BioPharm International 33, 17-20 (2020).
- BioPhorum, “An economic evaluation of buffer preparation philosophies for the biopharmaceutical industry” (2019). Available at https://bit.ly/3GDKOUN
- BioPhorum, “Nimble-Biophorum buffer stock blending system: A more advanced concept for buffer manufacturing” (2019). Available at https://bit.ly/3GJpgX6
Senior Vice President, Research & Development at Avantor
Manager, New Product Development, Avantor
Manager, Process Development, Avantor
Manager, R&D, Avantor



















