Effective Removal of Endotoxins with AEX RESIN TOYOPEARL® NH2-750F

contributed by Tosoh |
Endotoxins are remnants of bacterial cell walls that may contaminate drug products and cause an immunogenic response. They are often referred to as “pyrogens” due to their fever-inducing effects. Endotoxins may be found in drug products either due to contamination from host cells used to produce a drug product in a bacterial expression system or due to adventitious bacterial contamination in non-microbial products. Thus, endotoxin clearance is a requirement of downstream processing of biologics, especially those derived from microbial expression systems that contain endogenous host cell endotoxin. In this study, we evaluate the ability of TOYOPEARL NH2-750F for the removal of endotoxins by anion exchange chromatography.
Introduction
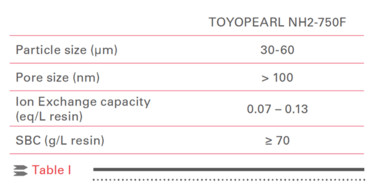
TOYOPEARL NH2-750F, a salt-tolerant anion exchange resin for process scale applications, is based on the TOYOPEARL HW-75F size exclusion resin functionalized with primary amine groups. Table I lists the properties of the TOYOPEARL NH2-750F resin.
TOYOPEARL NH2-750F resin is ideal for the intermediate purification of mAbs and other proteins where aggregates and other negatively charged impurities, such as DNA and endotoxins, are removed from the target of interest without having to dilute or buffer exchange the product prior to loading.
The data presented here demonstrate the capabilities of TOYOPEARL NH2-750F to remove endotoxin in a flowthrough chromatography process.
Experimental Conditions
An ÄKTA® system was flushed with 75 mL of 0.1 mol/L phosphoric acid, followed by 75 mL of E-pure water. The system was then flushed with 0.5 mol/L sodium hydroxide and incubated for a minimum of 1 hour at ambient temperature. Following the incubation, the system was flushed with 75 mL of E-pure water, and then with 20 mmol/L Tris base, pH 7.4, until a stable conductivity and pH baseline was noted. Adequate system cleaning was verified by Limulus amebocyte lysate (LAL) assay.
Log in or register to read this article in full and gain access to The Medicine Maker’s entire content archive. It’s FREE!



















