Multi Column Chromatography: Number of Columns Required for Optimizing Capacity and Productivity

contributed by Pall Biotech |
Xhorxhi Gjoka, Mark Allen Pagkaliwangan, Jonathan Hummel, Aditya Utturkar, Rene Gantier, Mark Schofield
Introduction
Over the last decade, advances in the upstream processing of monoclonal antibodies have resulted in higher bioreactor titers. With increasing titers, the production burden has shifted to downstream processing. Hence, the biopharmaceutical industry has reached a tipping point where the need for higher throughput in downstream processing is leading to the adoption of more efficient multi-column continuous counter-current chromatography systems capable of significantly reducing consumable cost.
In this study, the Protein A mAb capture process is modeled with a focus on the benefits of multi-column chromatography vs. single column (batch) operation. Here we explore the relationship between operating binding capacity (g mAb/L sorbent), residence-time, productivity (g/L/Hr), and the total number of columns required for optimal operation across a range of feed titers (2 g/L, 5 g/L, and 9 g/L).
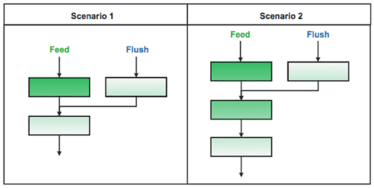
Figure 1: Common BioSMB column configuration scenarios during the load step. In scenario 1, two columns are loaded in series and the mobile phase from a previously loaded column is flushed onto the second pass column. In scenario 2, three columns are loaded in series and the mobile phase of a previously loaded column is flushed onto the second column.
Log in or register to read this article in full and gain access to The Medicine Maker’s entire content archive. It’s FREE!



















