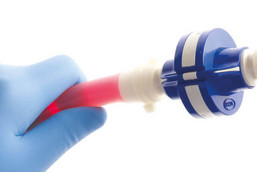
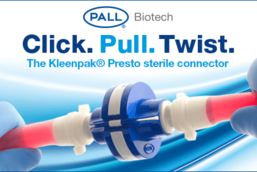
Pall Biotech
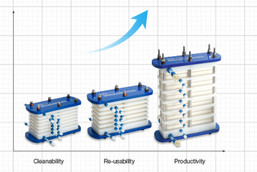
Pall Life Sciences, a division of Pall Corporation (a Danaher company), delivers total product and service solutions to global researchers, developers and manufacturers of large and small molecule drugs. The unmatched portfolio of traditional and single-use products is backed by custom service support and is both scalable and easily integrated to support processes from R&D through to commercial phases of production, including formulation/filling. As a core driver of the continuous bioprocessing movement, Pall Life Sciences has remained focused on delivering innovative semi- and fully continuous solutions, with the goal of continuously improving bioprocesses for a safer, healthier future.