Freeze-Dried Pharma
Lyophilized gene networks synthesize biomedicines on-demand – with just a sprinkle of water
Large-scale centralized manufacturing has helped bring cheaper pharmaceuticals to millions of patients, but for those in remote locations, access remains a problem – especially for medicines that require a cold chain. Increasingly, however, scientists are looking for ways to circumvent the problem by manufacturing drugs at the point-of-care.
In 2014, James Collins, a faculty member at the Wyss Institute at Harvard University and the Henri Termeer professor of medical engineering and science at Massachusetts Institute of Technology, and his colleagues developed a method for producing therapeutic molecules on-demand with freeze-dried synthetic gene networks (1) – the reaction is kick-started simply by adding water. Earlier this year, they used the technology to develop diagnostics that could detect Zika viral RNA at clinically relevant concentrations (2), and now, in their most recent study, they have used the technology to produce complex biomedicines (3). We asked Collins to tell us more.
How is it possible to synthesize biomedicines on demand?
The core aspect of our on-demand platform is taking the essential biological machinery in living cells that are able to generate biomolecules, extracting them, and placing them in a state of suspended animation by freeze drying. They can be stored at ambient temperatures for up to a year (as we have so far tested), but when water is added, all of the biosynthetic machinery – the ribosomes, RNA polymerases, and the dozens of enzymes responsible for protein translation and energy production – are reactivated. In the presence of the molecular manufacturing instructions (encoded in DNA), synthesis of the desired protein or peptide product begins.
What biomolecules have you experimented with so far?
We have demonstrated the production of four different vaccines, 10 different antimicrobial peptides, and 15 different antibody mimetics. These 15 antibodies were designed so that we could attach one of six functional domains covalently to create as many as 90 different antibody fusion proteins. We’ve also recapitulated a five-enzyme pathway that can make five related small molecules, including an antimicrobial called violacein. The limitations related to production are highly dependent on the specific therapeutic. In some cases, optimization was required to maximize yield, but essentially if you can find or design the necessary proteins, and express them in vitro, those products have the potential to be manufactured portably.
How difficult will it be to scale up the technology for real world applications?
We are working on this right now. Optimizing the yield will be key for practical deployment. We are currently testing our platform for conditions and strategies that maximize product production. One important consideration is that different therapeutics may require different levels of production. For example, molecules like vaccines may not require significant scale up, while other applications that require a larger therapeutic dose, such as antibody-based drugs, would likely require additional scaling. In our recent work, we scaled the production of the diphtheria vaccine to 33 human doses at a cost of around $10-18 per dose.
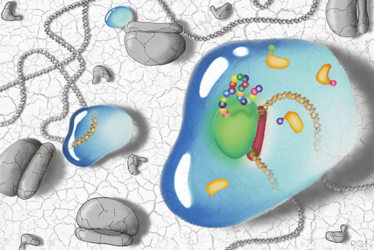
Molecular components for protein expression such as DNA and ribosomes can be freeze-dried, preserving their activity in a dormant state. Upon the addition of water, as shown in the droplets, the molecules are revived and the molecular instructions encoded on the DNA are translated and produced. Illustration by Peter Q. Nguyen
How much attention have you paid to cost?
For the examples we have calculated so far, costs are close to conventional methods. It is also likely that costs will drop as the field of cell-free biology continues to develop. In addition to direct production costs, the potential of this approach to lower the cost of distribution and storage was a main theme in our work. This includes no need for cold-chain transport for distribution; point-of-care does not have to have refrigeration infrastructure; small scale production could allow for the production of single doses, reducing the risk of waste and spoilage; and freeze-drying reduces the bulk of the mass for transport.
What impact do you think your work will have on the whole industry?
We think our platform could complement the current centralized production system. This is especially true for the distribution of protein-based drugs that require refrigeration or perhaps small-batch applications for personalized medicine. Having an easily stored, cheaply deployed, and on-demand manufacturing capability also has the potential to revolutionize access to advanced protein-based drugs for the developing world.
- K Pardee et al. “Paper-Based Synthetic Gene Networks”, Cell, 4, 940-954 (2014).
- K Pardee et al. “Rapid, low-cost detection of Zika virus using programmable biomolecular components”, Cell, 165, 1255-1266 (2016).
- K Pardee et al. “Portable, on-demand biomolecular manufacturing”, Cell, 167, 248-259 (2016).

Over the course of my Biomedical Sciences degree it dawned on me that my goal of becoming a scientist didn’t quite mesh with my lack of affinity for lab work. Thinking on my decision to pursue biology rather than English at age 15 – despite an aptitude for the latter – I realized that science writing was a way to combine what I loved with what I was good at.
From there I set out to gather as much freelancing experience as I could, spending 2 years developing scientific content for International Innovation, before completing an MSc in Science Communication. After gaining invaluable experience in supporting the communications efforts of CERN and IN-PART, I joined Texere – where I am focused on producing consistently engaging, cutting-edge and innovative content for our specialist audiences around the world.



















