Personalized Medicine × Nanotechnology
The combination of genomic profiling and high-tech targeted drug delivery is opening up a new front in the fight against cancer.
Delivering cancer drugs is a considerable challenge. Many exciting new drugs fail because they prove difficult to deliver safely and effectively. Some may be too toxic in their active form to be delivered systemically, while others are unstable, breaking down rapidly in plasma or not reliably taken up by cells, so that they never reach the tumor at all. Nor are existing drugs being used to their full potential – cancer chemotherapeutics are like a shotgun, mowing down healthy and cancerous cells alike, when what we need is a sniper rifle. Nanotechnology is one way we can improve our aim.
Many cancer drugs act on pathways or receptors found only in a subset of patients. On average, only 25 percent of patients respond to a given cancer drug, compared with 50 or 60 percent of patients receiving asthma or diabetes medication. Clearly, to avoid putting the patient through unnecessary treatment and maximize their chances of a cure, we need to apply personalized medicine – choosing drugs to match the individual circumstances and molecular profile of the patient.
My lab optimizes drug delivery by combining personalized medicine and nanotechnology to create therapies that are specific to the patient and their disease. Nanotechnology can provide the precise targeting we need, while personalized medicine ensures we’re using the right therapeutic bullets.
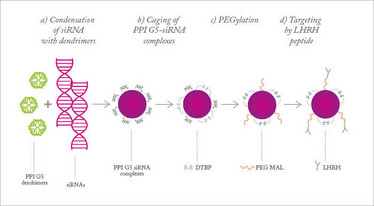
Figure 1. Nanoparticles for siRNA delivery. Adapted from (1).
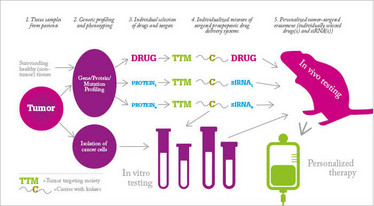
Figure 2. Personalized nanomedicine for ovarian cancer. Adapted from (3).
Dying by inches
Even if a drug proves effective at first use, there is no guarantee it will remain so. Even after months or years of remission, cancer patients face the prospect of relapse. For some cancers, it is a virtual certainty. What’s more, the cancer often comes back stronger, with resistance to the treatments that previously kept it at bay. How do cancer cells achieve this feat? Our cells are constantly bombarded with toxins, from our own metabolic processes and our environment. Luckily for us, evolution has furnished our cells with the means to adapt and survive, by producing proteins that help expel the toxins from the cell and repair any damage caused. Over time, the body builds up tolerance or resistance to the toxin – an effect often observed in regular alcohol drinkers, for example. Tumor cells retain these mechanisms, and even if only a tiny proportion of cells are resistant, this small population will survive the therapy and form a new tumor mass. Many drug regimes are eventually rendered ineffective by such mechanisms.
This process can be seen clearly in ovarian cancer. The disease is often diagnosed at an advanced stage, having already spread to neighboring or distant organs. Surgery alone cannot strip out all of the cancerous tissue in these patients so chemotherapy is given to kill the remaining tumor cells. There are various regimens available that may shrink the tumors temporarily, but most patients will relapse repeatedly over the course of months or years, as the cancer becomes resistant to each therapy. Eventually, we run out of options – one reason why five-year relative survival rates for ovarian cancer remain below 50 percent.
The ability of cancer to evade our therapeutic arsenal may also be related to the presence of cancer stem cells (also known as cancer-initiating cells). The malignant version of normal adult stem cells are believed to possess properties that make them more resistant to toxins, including anticancer drugs, and may constitute a perpetually renewing reservoir of malignancy. Thus, even if the rest of the tumor is destroyed, these phoenix-like cells can survive and generate a new drug-resistant tumor. Many labs are working to develop cancer stem cell-specific therapies that could be administered alongside existing chemotherapy drugs. If we could destroy cancer stem cells – the theory goes – we might be able to defeat the cancer once and for all, and halt the cycle of relapse and remission. We decided to develop a delivery system that could combine existing anticancer drugs with novel biologic therapies targeting cancer stem cells in ovarian cancer. By blocking the resistance mechanisms that cancer stem cells rely on, we hope to render them susceptible to established chemotherapy.
Shooting the messenger
Our approach to targeting cancer stem cells is to harness a natural mechanism for posttranslational gene silencing – RNA interference. Small fragments of RNA intercept mRNA and block translation of the gene into protein. Small interfering (si)RNAs have the advantage of being very specific and, unlike conventional monoclonal antibodies, can target intracellular proteins. But while they are a popular laboratory tool for gene silencing, they are naturally ill-suited to therapeutic use. Designed for life inside the cell, once in the harsh extracellular environment, they are degraded by serum nucleases within minutes and quickly removed by the kidneys. Even if they survive long enough, they are unable to escape the blood stream unaided, as they cannot easily cross the cell membranes.
We needed a delivery system that could safely and simultaneously transport both cytotoxic chemotherapy drugs and delicate siRNAs to the site of the tumor and release them inside the cells. Nanoparticles were the obvious choice. Nanoparticles for therapeutic use come in many forms, depending on the route of administration and where in the body you want them to end up. In this case, previous research told us that nanoparticles consisting of star-shaped polypropylene-imine (PPI) dendrimers were preferential for delivering siRNAs intracellularly, and could also be used for targeted delivery of platinum-based chemotherapy drugs. To maintain stability in the inhospitable environment of the blood stream, the nanoparticles were “caged” with a dithiol-containing cross-linker and coated in PEG polymer (see Figure 1) (1). PEG provides a good anchor for the targeting molecule, synthetic luteinizing hormone-releasing hormone (LHRH), which is massively overexpressed in ovarian cancer cells.
For our initial experiments, we used siRNA targeting the CD44 molecule, a marker expressed in putative ovarian cancer stem cells. In mice given human ovarian cancer xenografts, we found that the siRNA nanoparticles successfully suppressed CD44 protein. The combination therapy enhanced antitumor efficacy of an anticancer drug, limited tumor growth and reduced side effects compared with standard chemotherapy alone (2). However, this is by no means a universal treatment. CD44 is just one of an array of proteins that impact on drug resistance in ovarian cancer, which vary from patient to patient – there is no guarantee that a given tumor would respond in all patients if we suppress just CD44 protein. If we only target one protein, resistance would surely develop, just as it has to conventional drugs.
Our proposed approach combines personalized medicine and nanotechnology to deliver a panel of therapies based on the genotype and phenotype of tumor tissue (see Figure 2). Following first-line surgical treatment, tissue samples from the tumor and healthy tissue of ovarian cancer patients will be compared to identify the key targets. At the same time cancer cells will be isolated and cultured for in vitro testing. These data will allow us to come up with a tailored treatment plan, comprising a panel of siRNAs and cytotoxic drugs, all delivered directly to the remaining cancer cells by LHRH-targeted nanoparticles. In mice, this approach dramatically reduces tumor volume compared with conventional platinum-based chemotherapy (3). We believe this approach could offer hope to patients with very few remaining treatment options and we are keen to move the treatment into clinical trials. Unfortunately, nanotechnology doesn’t come cheap, and even a small Phase I clinical study can cost a million dollars. It’s not easy finding partners with that kind of money who are willing to take a risk on something new, and even then, it’s a long path ahead to the clinic. But I remain hopeful for the future. Nanotechnology is a natural partner to personalized medicine and together I believe they can revolutionize cancer treatment.
Tamara Minko is a Distinguished Professor and Chair of the Department of Pharmaceutics at Rutgers, The State University of New Jersey, USA. First published in The Translational Scientist, a sister publication to The Medicine Maker.
- O. Taratula, O. B. Garbuzenko, P. Kirkpatrick, I. Pandya, R. Savla, V. P. Pozharov, H. He, T. Minko, “Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery”, J. Control. Release. 140(3), 284–293 (2009).
- V. Shah, O. Taratula, O. B. Garbuzenko, O. R. Taratula, L. Rodriguez-Rodriguez, T. Minko, “Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug”, Clin. Cancer Res. 19(22), 6193–6204 (2013).
- T. Minko, L. Rodriguez-Rodriguez and V. Pozharov, “Nanotechnology approaches for personalized treatment of multidrug resistant cancers”, Adv. Drug Deliv. Rev. 65 (13–14), 1880–1895 (2013).



















