
Riding the Cell Therapy Wave
A surge of exciting new cellbased treatments is coming – but is the industry ready to manufacture them and catch the wave to success?
It is clear that the manufacture of patient-specific cell therapies (PSCTs) involves unprecedented complexities. The most obvious challenge is that PSCTs, by their very nature (cells are extracted from a patient or a matched donor, processed and then returned to the patient), demand that an individual cell therapy product is made for each individual, eliminating all the efficiencies associated with traditional high-throughput drug manufacture. Furthermore, the process also differs from the manufacture of “normal” biologics on many levels, including regulatory, supply chain logistics, complexity of product attributes and the complexity of preserving and storing therapies composed of living cells. The good news? The cell therapy industry has evolved; developers, manufacturers, and regulators have all become better at addressing many of the aforementioned challenges. By working together and thinking laterally, these parties are devising innovative solutions that will make novel medical treatments a reality. But one key challenge still looms ahead: commercial-scale manufacturing.
Current PSCT production processes require an abundance of cleanroom space, operational expertise and expert personnel, and incur significant overhead costs. As a consequence, current cell therapy manufacture is generally not viable at a commercial scale.
Nevertheless, projections from Informa and the Alliance for Regenerative Medicine suggest that a tsunami of potential PSCT products is approaching: in 2014, there were 378 regenerative medicine trials (39 in Phase III and 206 in Phase II), and by late 2016, that number rose to 801 (68 in Phase III and 467 in Phase II) (1). Clearly, there are good reasons to address the scalability issue.
I covered the manufacturing problem in a previous article (2). Here, I’d like to offer practical advice that forms part of the solution.
Avoiding wipeout
For cell therapy manufacturing to be commercially successful, a systematic development paradigm should be used; Development by Design addresses quality, cost of goods (COGs), scalability, and sustainability – failure to balance any one of these elements may lead to a “wipeout”. Automation – along with other approaches, such as integration, elimination, simplification, and sharing (see box, Automation Strategy) – can help optimize many of these factors.
Quality
Quality is foundational for all therapeutics. However, the manual, open, and human-dependent nature of many PSCT process steps can make it difficult for manufacturers to meet the critical quality attributes (CQAs) of the final product. And it is often impractical to perform the complete range of lot-release tests required to confirm that all CQAs are met for each PSCT lot. How can we develop a robust process that can consistently produce high quality material? Well, automation (and related process optimization) can improve quality in at least two ways. First, automation significantly reduces the risk of human errors, such as mistakes in data recording/calculations or errors in the performance of manual process steps. Second, it reduces process variability. Despite excellent training and experience, human labor is by nature variable (individuals may perform a given task differently to others or may not perform a task consistently from one day to another). Reducing variability (through automation and optimization) results in more reproducible processes.
COGs
The high COGs of PSCT products (typically driven by labor and testing costs) demand a proportionately high commercial value proposition. As clinical development progresses and clarity around the actual value proposition builds, it is critical to optimize commercial viability by focusing on COGs reduction. COGs minimization depends on many factors, including choice of raw material supplier, the decision to manufacture in-house or to outsource manufacture and, of course, where and how to invest in automation. As one example, an early cell therapy product was launched using a manual manufacturing process, with the aim of introducing cost-saving automation later. However, lower than anticipated demand for the therapy led to excessive COGs after launch. The manual nature of the process led to a relatively high direct cost of the product, resulting in significant problems for the cell therapy developer. An appropriate investment in automation prior to commercialization might have potentially reduced both direct and overhead costs, and better positioned the therapy for long-term commercial success.
Automation reduces the cost of manual labor (by cutting both the number of hours and the level of expertise needed), the cost of equipment and associated consumables (if several unit operations can be handled by one piece of equipment), and overheads (by saving on manufacturing time and space requirements). Labor costs, in particular, can have a significant impact on cell therapy COGs. Though automating one unit operation may only save one hour of labor per product batch in traditional pharmaceuticals (where one batch provides treatments for thousands of patients or more), with PSCTs, because one batch represents one patient, the hour of labor is saved over and over again.
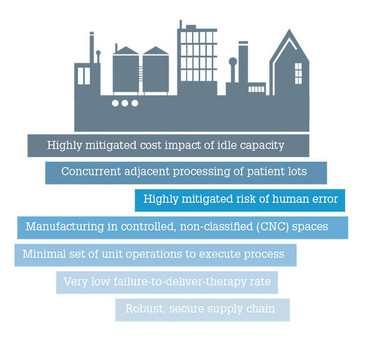
Figure 1. Idealized future state of PSCT manufacture.
Automation Strategy
Automation strategy needs to address a range of considerations, including:
- process automation (closed-loop process control)
- task automation (for example, selection process, wash and formulate process)
- test automation (for example, compendial method)
- factory automation – information (electronic batch records) and execution (manufacturing execution systems or MES).
Automation is one of several strategies for manufacturing optimization that also includes:
- sharing (performance of more than one unit operation by the same technology)
- integration (combining two or more unit operations into one)
- simplification
- elimination (identifying steps that are not necessary to meet the product’s required attributes).
One area of COGs that is often overlooked is the cost of adverse quality events, such as deviations and out-of-specification incidents. Each adverse quality event demands management and investigation, and the associated labor costs can rapidly escalate. In the extreme, an adverse quality event could result in a failed process that must be aborted, requiring that the sunk costs be absorbed as part of the COGs for successfully completed products. This is in addition to the wider implications of delay or failure to deliver the product, including impact on the patient, impact on accrual objectives and costs for clinical-stage programs – as well as diminished market confidence for commercial-stage programs. A well-executed plan for automation can substantially reduce the occurrence of adverse quality events and therefore eliminate the associated direct and indirect costs.
| Risk Level | Example | Timing to limit risk |
| None | Change to automated sterility test | Before BLA |
| Low | Change in process unit operation and “cell journey” is the same | Prior to 50 percent accrual in pivotal trials |
| Medium | Change in process unit operation and “cell journey” is similar | Prior to initiation of pivotal trials |
| High | Change in process unit operation and “cell journey” is modified | Some Phase II clinical data |
Table 1. Managing comparability risk.
Considering Comparability Risk
A good surfer understands that each time a new wave approaches, timing – derived from a balance of experience and instinct – is crucial. Act too soon or delay too long, and you will inevitably go under. Likewise, certain criteria in cell therapy manufacturing can guide our judgment about when to automate.
A developer must consider the potential comparability risk of making changes to automated processes early or late in clinical development, and anticipate the implications. As dictated by the FDA, cell therapy developers must demonstrate that any manufacturing change does not significantly affect safety, identity, purity, or potency. Depending on the nature of the change and the requirements of product characterization, this demonstration of comparability between pre- and post-change product may only require laboratory testing. At the other extreme, it may demand additional clinical studies. A change to the manufacturing process presents a comparability risk even when it occurs early in a clinical development program, but much less is at stake than when changes are made after substantial clinical data has been generated.
Some process changes are associated with relatively low comparability risks, while others have relatively high risks. For example, changing from a manual to an automated method in a core process step, such as changing from a manual static culture process to an automated perfusion bioreactor, would present a major comparability risk. By contrast, the risk associated with switching from manual record keeping methods to automated electronic record keeping is minimal. The risk is lower, generally, when the change does not alter the journey that the cells are on. Some examples of change-risk relationships are listed in Table 1.
Scalability
Crucial for the success of the cell therapy industry as a whole is the ability to migrate from a clinical-scale process, with the capacity to make tens to hundreds of patient doses per year, to a commercial-scale process that can provide thousands to tens of thousands of patient doses per year. For PSCT products, this means that the number of batches must increase. Treating exponentially larger numbers of patients necessitates exponentially larger staff. The challenge of recruiting and maintaining trained staff limits the rate at which scale can increase – and where process complexity is high, there may not be enough qualified labor in the local geographic area to meet demand. Therefore, automation will likely be essential for scale-up. Furthermore, automation usually involves closed-system design, which can substantially shrink the physical footprint of the facility, and simplify infrastructure (for example, controlled non-classified space instead of ISO 7 cleanrooms). The result is shorter timeframes and lower investment burdens.
Sustainability
Automation can solve many manufacturing challenges – but not all of them. Even if quality, COGs, and scale objectives are met, there is the danger that manufacturing cannot be sustained over the full product life cycle. A key risk is disruption of the supply chain, which for cell therapy is still immature and relatively fragile. In the worst case, where a process step relies on supply chain elements that become unavailable, continued production will require manufacturing changes to be developed, tested, and demonstrated to be comparable. In many cases, automation technology is proprietary to its developer, and presents a sole-source supply chain. In these instances, it is important to assess the risk of interrupted access to the technology, such as equipment, consumables, technical support and service, and to establish suitable risk mitigation strategies.
Although automation can present supply chain challenges, it can also improve sustainability, if applied carefully and strategically. For example, automating materials handling and logistics can help manage relationships with suppliers, particularly as scale increases towards commercial requirements, by providing the developer and supplier with real-time data on demand and supply.
In terms of commercial sustainability, monitoring process consistency over time will be critical. Automated collection of process data helps to efficiently track and identify trends in data, which can be extremely beneficial in, for example, ensuring that product specifications are maintained over time. Additionally, this type of automation can reduce labor requirements significantly compared to manual extraction of data from hundreds or thousands of batch records.
Automation: surf’s up?
I hope you will agree that we have established that automation is a key part of achieving commercially viable PSCT manufacture, but how do you choose solutions? A full knowledge of the landscape will ensure that you’re able to choose automation technology that exploits the best available solutions for specific process requirements. Let’s be honest: it’s not in anyone’s best interests to re-invent the wheel when a wheel works just fine! Unfortunately, there is still an unmet need for cell processing platforms that can perform a variety of cell manipulations across a range of scale – but innovation is starting to happen. For example, automated, programmable counter-flow centrifugation (CFC) platforms are being designed that can operate with single-use systems. Such platforms will help reduce the need for highly skilled staff and high-specification cleanrooms.
As mentioned earlier, despite the main advantages of automation, there are times when the justification – the return on investment – just isn’t there. Consider a switch to automation that costs $10 million to develop, but only saves $100 per product. If a cell therapy developer plans on making only a small number of treatments over the life of the product (many PSCTs target rare diseases, for example), then the return on investment of $10 million certainly does not justify the investment. It is crucial to strategically consider whether the upfront cost of automation will be balanced by later savings in cost, time and labor.
It is also important to bear in mind that automation is only as good as the programming and direction given. If an automated process blindly moves things along in assembly-line fashion, but cannot gracefully detect and respond to errors or failures, the perceived benefits could quickly be outweighed. If an automated process produces flawless product most of the time, but fails in one out of every 100 runs, then one out of every 100 patients cannot be treated. Not only does this have serious implications for the patient, it leaves a PSCT product that cannot be reimbursed.
To help inform automation decisions and other manufacturing considerations, it is important to work with a manufacturing team – either internally or at a contract manufacturing organization partner – to strategically plan for commercial manufacturing needs. Plans should include:
- A thorough breakdown of the current state of a developer’s product and process (for example, quality target product profile, CQAs, and unit operations).
- Analyses (for example, process capability analysis, quality risk analysis, COGs analysis, scalability analysis, sustainability analysis, technology and landscape analysis).
- A development and optimization strategy that provides a roadmap to a future commercial state. Planning allows the cell therapy developer to apprise and align its stakeholders, make informed choices about further development, prepare for future unit operations and project COGs both at commercial launch and post-launch.
For the cell therapy industry as a whole, and PSCTs in particular, to truly become commercially viable, we must envision and develop a radically different future state of manufacturing, which is likely to have at least some of the attributes noted in Figure 1.
Cell therapy manufacturing must largely move away from the cleanroom model and into the “back of the facility” – into production spaces more suited to high-volume production. That is not to say that cleanrooms have no place in cell therapy; however, whenever automation, integration, and closed processing systems result in a simpler manufacturing space that is used for multiple processes simultaneously, your bottom line will be healthier. And a healthier bottom line helps carve a world where cell-based therapies are accessible to all.
Brian Hampson is Vice President, Global Manufacturing Sciences and Technology, PCT, a subsidiary of Caladrius Biosciences, Allendale, NJ, USA.
- Alliance for Regenerative Medicine, “ARM Q3 2016 Data Report”, (2016). Available at: bit.ly/2gwciBb. Accessed December 12, 2016.
- B Hampson, “Brave New Factories”, The Medicine Maker, 25, 40–42 (2016). Available at: bit.ly/2h23McI



















