
The Beauty of the Box
Cytiva’s KUBio box modular environment for Viral Vectors took the top spot in The Medicine Maker 2019 Innovation Awards. Here, we explore why the industry is so excited about modular bioprocessing capabilities.
Nominations for The Medicine Maker 2020 Innovation Awards are now open. Click here
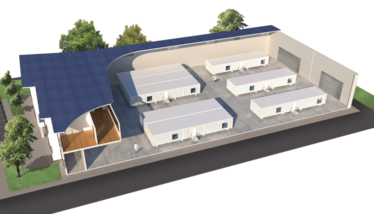
It has often been said that pharma is slow to change, but when it comes to biologics that notion does not hold true. Bioprocessing is no longer about just monoclonal antibodies – cell and gene therapies have emerged as one of the hottest topics in the industry. The COVID-19 pandemic is also driving many companies to evaluate new approaches to vaccine manufacture. Bioprocessing is becoming more diverse and companies require flexible facilities that allow them to adapt to changing demands for different products.
Cytiva developed its KUBio facility to help companies quickly deploy new manufacturing capabilities. A KUBio facility is made up of modular units, allowing for design flexibility – and also offering the capability for companies to expand further as manufacturing demand rises. For smaller projects, the company also offers a modular environment, called the KUBio box, which is designed to fit into existing facilities or newly constructed shell buildings. The latest version – the KUBio box for Viral Vectors – is, as you may have guessed, specifically designed for viral vector manufacture, and is based on single-use technology embedded within a biosafety level 2 (BSL-2) environment.
We get the development story behind the Box from Olivier Loeillot, Senior Vice President, Bioprocessing at Cytiva, who is considered the father of the KUBio concept.
How did KUBio begin?
Back in 2010 when I was working for Lonza, I bumped into Kieran Murphy, then CEO of GE Healthcare, at an airport. We got talking and soon realized we had similar ideas – we both wanted to move from providing consumables and hardware to providing complete manufacturing plants. The idea was to combine many products and services into a single offering.
When I subsequently joined GE, Kieran and I had to start from scratch; making our chat a reality by devising a product that customers would be interested in was not going to be easy. We only had a tiny team, but we could see that pharma companies wanted to move from costly, stainless-steel plants towards smaller, more agile, and less costly solutions – without compromising quality. At the same time, the biopharma industry was also beginning to take shape in China and India, and there was huge demand for high-quality facilities that could be built faster and more cost effectively than traditional stainless steel plants. Foreign companies were also looking to create regional capacity in these countries quickly.
There was also rising interest in single use technology, which informed our acquisition of Xcellerex in 2012. We designed the first KUBio in 2012 – which included single-use technology (our FlexFactory biomanufacturing platform) as a key technology.
What challenges did you face in development?
Other companies have previously attempted to introduce modular bioprocessing solutions, but many have failed. When we were designing KUBio, we had a simple goal: figure out the problems and remedy the causes. Our analysis suggested that previous failures were likely due to insufficient appreciation of the importance of localization. Non-localized manufacture of a modular facility, followed by export – and the associated transport expenses, shipping time, import taxes, and so on – will rapidly erode the time and cost advantages of a modular approach. And that’s why we opted for local manufacture of KUBio with partner companies.
Another challenge was the perception of modular plants – some saw them as low-cost, low-quality options. Accordingly, we applied state-of-the-art quality standards to modular construction. I think the results are clear; KUBio plants have been running for six years now without problems. Modular plants are evidently not inferior to conventional plants.
What types of companies adopt KUBio?
At first, we thought the KUBio concept would mainly suit small biotechs in emerging markets – those companies that need support and expertise to establish a manufacturing plant. JHL Biotech Inc. (now Chime Biologics) was one of our early customers. They wanted to get ahead of the market and establish a new facility in China – and we were able to support them. Similarly, we helped another Chinese company, BeiGene, to evolve from manufacturing biosimilars to making innovator biologics; KUBio and FlexFactory were key in this endeavor. Today, BeiGene is a global name with several big pharma collaborations.
We were surprised, however, to see that modular manufacturing was also of interest to big pharma. Recently, for example, we designed a state-of-the-art biosimilars plant for Pfizer in China. We also supported the initiation of Pfizer’s Chinese operations. We expect to see products from Pfizer’s KUBio plant on the Chinese market very soon. And now we are setting up a Chinese manufacturing plant for Lonza, which should be operational in 6-12 months.
How does the KUBio box differ from KUBio facilities?
The Box is designed to fit inside an existing building; its footprint is only 800–1,000 square meters. The original KUBio facility is two or three times larger and can sit outside. Deploying a Box allows companies to easily add new capacity or repurpose existing facilities at a fraction of the cost of an entirely new plant. Because the boxes are small, they allow for high flexibility. For example, one client has requested a triple KUBio box installation incorporating a cell therapy KUBio, a gene therapy KUBio and a fill-and-finish KUBio box – all next to each other in a single shell. The Boxes are suitable for small volume manufacturing – and there is increasing demand for this in the industry. Because of the smaller footprint, however, the Box does not incorporate manufacturing peripherals or process liquids. The assumption is that clients will already have these capabilities and resources.
How has the KUBio concept kept pace with market trends?
We started with KUBio for monoclonal antibodies, but in recent years we have developed a BSL-2 KUBio, the Cell Therapy KUBio for CAR-T products, and now we’re deploying the KUBio box for viral vectors. The platform is particularly important at present because of the crippling lack of capacity in the gene therapy manufacturing sector – it is not uncommon for biotechs seeking CDMO-mediated manufacture of their viral vector to have to wait 18 months for a manufacturing slot! Such companies urgently require an option that gives them manufacturing autonomy. More specifically, the increasing interest in cell and gene therapy approaches and personalized medicine is driving the increased diversity of biologics and smaller patient numbers per drug. This, in turn, demands approaches that permit manufacture at lower cost and smaller volumes, while retaining the flexibility to rapidly add capacity as required. Modular bioprocessing systems are simple, fast to implement, and very cost-competitive – crucially, without compromising on high quality standards.
What is your view on the future of biologics manufacture?
I think that bioprocess efficiency can be improved by advanced automation – and this is something we’ll be focusing on at Cytiva. We’ve signed an agreement with Rockwell for access to their PlantPAx distributed control technology, which we intend to incorporate into our product range by 2021. We are also working on digital systems that will enable intelligent manufacturing plants that can analyze historical batch data and use artificial intelligence tools to predict outcomes for the in-process batch. These capabilities will also identify key process parameters driving yield or productivity. Ultimately, our goal is for a fully intelligent, digital KUBio. Operators in any part of the world will be able to control multiple KUBio-mediated bioprocesses anywhere else in the world.
More generally – partly due to the COVID-19 pandemic – I believe we will increasingly see countries seeking pharmaceutical autonomy; governments will prioritize localized bioprocessing capabilities so that they have the ability to manufacture treatments for their own populations. Similarly, manufacturers will want the ability to rapidly start the manufacture of a new drug or vaccine and quickly scale up. Modular solutions can meet these goals – and a number of governments have already contacted us to discuss how their countries can become more independent and agile when it comes to drug and vaccine manufacture.
Challenges and Opportunities in Viral Vector Manufacture
Joe Makowiecki has worked in the bioprocessing field for 25 years, and now oversees the deployment of Cytiva’s KUBio facilities, KUBio box systems, and FlexFactory single-use biomanufacturing platforms
I’m very excited to be working in the advanced medicine field. These therapies could have a tremendous impact on human health, but the field is not yet mature and needs a little extra help. Viral vectors today are in a similar position to monoclonal antibodies a decade or two ago, but viral vectors have many unique challenges. Viral vectors are relatively unstable and their complex structures are susceptible to degradation by shear, pH, and temperature. Furthermore, operator safety demands relatively high safety standards – BSL-2 rather than BSL-1 – which places constraints on the design and construction of the manufacturing plant. Essential safety features include adequate segregation, single-pass air systems, and carefully designed workflows. Elsewhere, there is still room for improvement in various areas, including virus production and viral titer optimization, as well as in downstream processes, such as vector recovery. Processes and technologies will continue to improve and evolve as the industry gains experience with viral vector manufacture – and I hope that our KUBio product platform will reflect the industry’s evolution by helping it to accommodate the exciting new therapies and processes we now see in development.
The gene therapy market is growing at about 34 percent per annum (compound) and there has been a huge increase in clinical trials. Many gene therapies rely on viral vectors, which explains the industry’s severe capacity deficit that Olivier alluded to. In many cases, CDMOs just cannot keep up with demand – and given how exciting these therapies are, it’s heartbreaking that companies have to wait so long to find capacity. We thought our KUBio concept would help plug the gap, which is why we adapted the technology to create the KUBio box for viral vector manufacture. The original KUBio concept was designed with simplification, standardization, speed to manufacture, and flexibility in mind. However, we realized it could be even more versatile, so we modified it to fit into shell buildings. The KUBio box takes our FlexFactory single-use biomanufacturing platform and surrounds it with a cleanroom environment. There are actually a lot of existing spaces in pharma facilities; the space may not be big enough to put in new stainless steel infrastructure, but it can be used to “drop in” a KUBio box – at most, the box requires a very simple shell building. The FlexFactory itself is also very configurable and allows a single KUBio box installation to manufacture multiple vector types, including lentivirus, adenovirus, and adeno-associated virus, and to rapidly scale production on demand. The beauty of the Box is that it is part of a standard platform as the components – upstream bioreactors and mixers, process liquid, chromatography systems, and tangential flow-filtration units – are similar to those used in other KUBio models. Standardization is considered crucial by many of our clients because they want to roll out local production for viral vector based therapies to help bring costs down. Centralized production is considered too expensive because of the transportation involved. Replicating a process for a complex CAR-T therapy is much easier if you have exactly the same equipment and set up at different sites. At the same time, clients can tweak the system in various ways to suit their exact process needs – we offer all kinds of a la carte buffer preparation and fill/finish options. Many clients appreciate this support; instead of struggling with many separate decisions regarding choice of GMP equipment, automation layer, consumable, and so on, they simply adopt an end-to-end, standardized single-use platform. We also take care of project management, delivery installation, commissioning, and qualification. KUBio is truly turnkey.
In my view, nothing beats modular systems in terms of speed and predictability. They are built inside so progress is fast and there is far greater control. With conventional stick-built facilities, on the other hand, there can be labor- and weather-related delays, and issues with consistency of materials.

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















