
When Standardization Meets Customization
To bring your biologic to market as quickly as possible, streamlined scale up is essential. By combining standardized, single-use platform technologies that can be customized to your particular process requirements, you can unlock crucial efficiency gains.
Jeremy Rautenbach |
sponsored by Pall Biotech
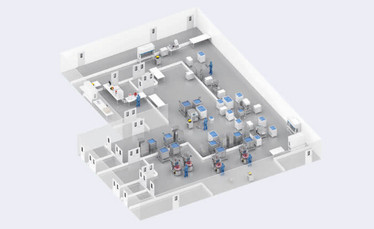
Figure 1: A 3-D representation of Pall Biotech's Gene Therapy platform in the Pall Solution Builder environment.
Pharmaceutical companies can spend upwards of $2 billion developing a drug (1), but by the time it is ready to be commercialized, more than half of its patent exclusivity period is likely to have elapsed. When you also consider that only a small minority of the Phase I drugs reach the commercial scale (around 14 percent) (2), companies must take full advantage of their marketable drugs to recover their investment costs – this means getting to market as quickly as possible upon receiving Phase III approval.
An important factor in maximizing speed to market is scale up. During Phase II and III studies, companies will be manufacturing small quantities of clinical material and, typically, using equipment that will not support anticipated drug demand during commercialization. Scaling a bioprocess to accommodate larger flow rates and higher output volumes can be a lengthy and sometimes difficult task. Many of the challenges are as a result of making trade-offs between the availability of fundamental technologies (such as instrumentation, pumps and tubing) and optimally operating processing equipment within their designed ranges – this can be a fine (and time consuming) balance.
As well as expertise, a broad technology portfolio including single-use, stainless steel or continuous technologies ensures a choice of manufacturing methods are available to suit. Pall Biotech has such a portfolio of technologies and has further refined its offering by designing standard mAb and gene therapy processing platforms to assist clients with their scale-up requirements. These platforms include all hardware, consumables and interconnecting manifolds, ready-to-go. Development of such platforms has, for example, enabled Pall Biotech to reduce consumables part counts by 30 percent, ultimately translating into reduction in warehousing, increased supply chain flexibility and reduced lead times for clients. However, should these designs not fit the process requirements, they are flexible enough to be configured with relative ease (see our sidebar for more on what “standard” really means).
These hardware systems need to be specified correctly, integrated thoughtfully into the facility, and controlled with the appropriate level of automation to operate efficiently and safely. There are a number of questions that require careful consideration. For example, what equipment is required to support the bioprocess? What are the most optimal process conditions? What is the footprint required to operate efficiently, and what is the associated material and personnel flow within the facility? What is the best method of controlling the equipment, and how will it integrate into the facility’s automation architecture?
Biotech Integrated Solutions
Carefully answering these questions and translating process requirements into the correct bioprocessing technologies requires a team of project, design, automation and process engineers, regulatory experts and validation scientists working together from the start. Speed, project management and process expertise are paramount for a successful scale-up to a commercial manufacturing facility, and Pall Biotech has structured its Biotech Integrated Solutions (BIS) team to bring the required expertise to partner with and support clients on their biotech journey.
Knowledge sharing
Before designing a solution, Pall Biotech’s team work with the client to fully characterize the process and understand specific needs. A deep dialogue upfront is invaluable and allows the Integrated Solutions team to fully appreciate all the process requirements and translate them into the appropriate technical solution. In our experience, this approach leads to significant savings during project execution because requirements have been well understood and clarified.
To further enhance this dialogue, the team utilizes an internally developed mass balance tool to assist with quantifying expected fluid volumes and product yields to inform system sizing and the equipment list. This tool, in combination with the client’s specific mass balance and process description, enables the team to compile a suitable equipment list from Pall’s technology portfolio. The design and process engineers have deep technical knowledge of Pall’s standard technology platforms that allow us to provide guidance on optimal operating ranges, but also identify where equipment may need to be modified to meet individual process requirements.
Solution design
Once the equipment list has been finalized, the Pall Solution Builder platform is used to construct a 3-D model of the client’s facility which helps to visualize the intended process flow. The 3-D model helps to confirm the equipment will fit in the facility, provide an appreciation of material and operator flows, and demonstrate the interconnectivity of the equipment and ancillaries. The integration of the equipment into the facility is oftentimes overlooked when generic solutions are proposed, and only discovered during the project execution phase leading to significant rework.
After the equipment and layout have been confirmed, specifying the single-use manifolds, transfer sets and biocontainers can start. Leveraging the existing consumable designs from Pall’s mAb and gene therapy platforms and incorporating them into the Solution Builder’s 3-D environment, the team will be able to quickly identify any customizations required.
Then, with an approved single-use system, the discussion around automation and control can begin. This conversation is very important because every process and facility is unique. Some manufacturers will require local control of their systems, using PLC or an HMI on each piece of equipment. Others, however, may want to operate their equipment from a centralized node. There are a lot of nuances and it is incumbent on suppliers to build flexible technology platforms that can be modified to suit the company’s requirements; for example, larger biopharma companies will have internal automation and control teams. Pall Biotech is aware of these automation nuances and has built flexible technology platforms that can be configured to suit process requirements.
Project execution and delivery
In addition to building and qualifying the equipment, the Integrated Solutions team helps with site acceptance testing, factory acceptance testing, training, maintenance, regulatory guidance and any other service the client requires.
The multifaceted BIS team understand Pall’s systems in depth and can identify exactly where the processing limitations are and how they can be balanced to meet the client’s needs.
The advantage of choosing a one stop shop over a system integrator as your solution provider lies in the depth of process and technical knowledge that comes from years of designing and qualifying bioprocessing equipment. In short, we know exactly how our equipment works, where it performs optimally and how to configure it to suit the client’s process requirements.
Transferring a drug to commercial scale manufacturing at speed is however only one part of the success – the second is ensuring business continuity during the commercial phase. Pall Biotech has a long history of supporting customers during this critical time and has implemented robust supply chain processes assuring customers of supply continuity. Pall Biotech has extensive visibility into its supply chain ensuring and works proactively with its suppliers to ensure adequate risk mitigations are in place – from single-use components supply to irradiation capacity.
When taken forward by Pall, the time it takes to scale up a given process can be dramatically reduced. And the sooner a process is ready for commercial manufacture, the sooner the therapy is on the market treating patients, and the sooner the companies can develop more life-saving medicines.
A Servier of Success
Recently, Pall announced that Servier has selected them as an exclusive technology and services provider for their new site in Gidy, France, which will develop and manufacture monoclonal antibodies and recombinant proteins. This supply includes a complete, end-to-end bioprocessing solution from upstream to bulk drug substance.
“We selected Pall Biotech as our single supplier because of their capacity to deliver the complete suite of development and manufacturing equipment, including an automation package, assurance of consumables supply and technical support under a well-defined project execution plan,” said Renaud Bessière, Director of the Bio-S Project at Servier in a press release (4).
The facility is part of the Bio-S project at Servier, which is focused on delivering best-in-class support for oncology drug production from R&D to clinical scale. The Bio-S unit will include a development workshop dedicated to monoclonal antibody processes, which will be operational by the end of 2019. The rest of the unit – combining all of the steps necessary to deliver an injectable product to humans – will be operational in 2020.
- JA DiMasi, HG Grabowski and RW Hansen, “Innovation in the pharmaceutical industry: New estimates of R&D costs”, J Health Econ, 47, 20-33 (2016). PMID: 26928437.
- CH Wong, KW Siah and AW Lo, “Estimation of clinical trial success rates and related parameters”, Biostatistics, 20, 273-286 (2019). PMID: 29394327.
- Pall Biotech, “Standardizing Single-Use”, The Medicine Maker. Available at: https://bit.ly/2kqSaUp.
- Pall, “Pall Corporation chosen as single-source supplier by Servier” (2019). Available at: https://bit.ly/2Nozb9a.
Global Product Manager for Integrated Solutions at Pall Biotech.



















